The Medicines Patent Pool secured its first licence for malaria, tuberculosis and hepatitis C
On Sept. 21, 2021, the Medicines Patent Pool (MPP) announced that it had signed a licence agreement with…

On Sept. 21, 2021, the Medicines Patent Pool (MPP) announced that it had signed a licence agreement with…

On Sept. 16, 2021, Novavax announced its participation in a newly expanded Phase 2 clinical trial called Comparing…
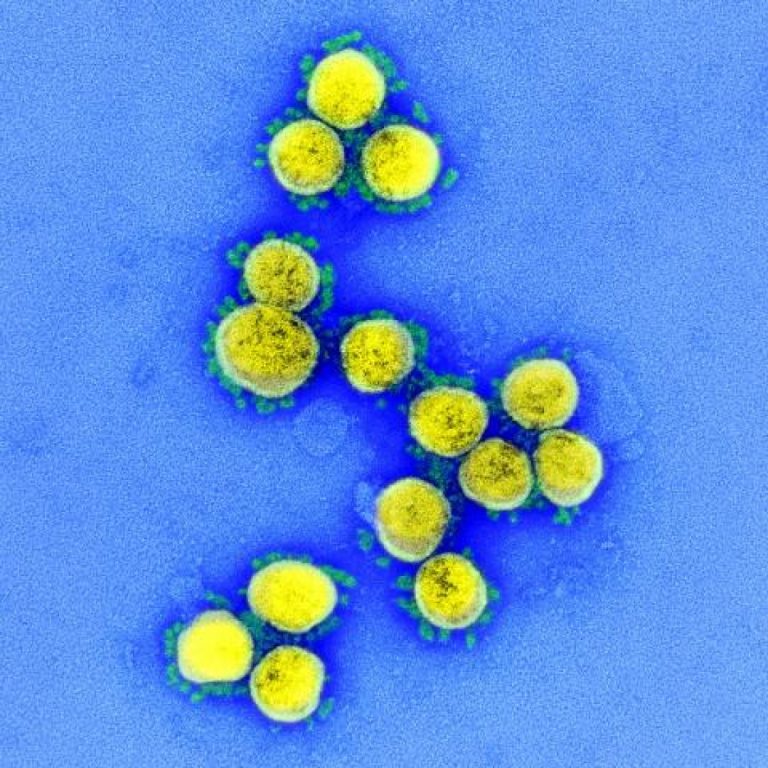
On Aug. 5, 2021, Novavax announced preliminary data demonstrating that a single booster dose of its recombinant nanoparticle…

On Aug. 4, 2021, Novavax announced that it had reached an agreement with the European Commission (EC) for…

On Jun. 14, 2021, Novavax announced that NVX-CoV2373, its recombinant nanoparticle protein-based COVID-19 vaccine, demonstrated 100% protection against…
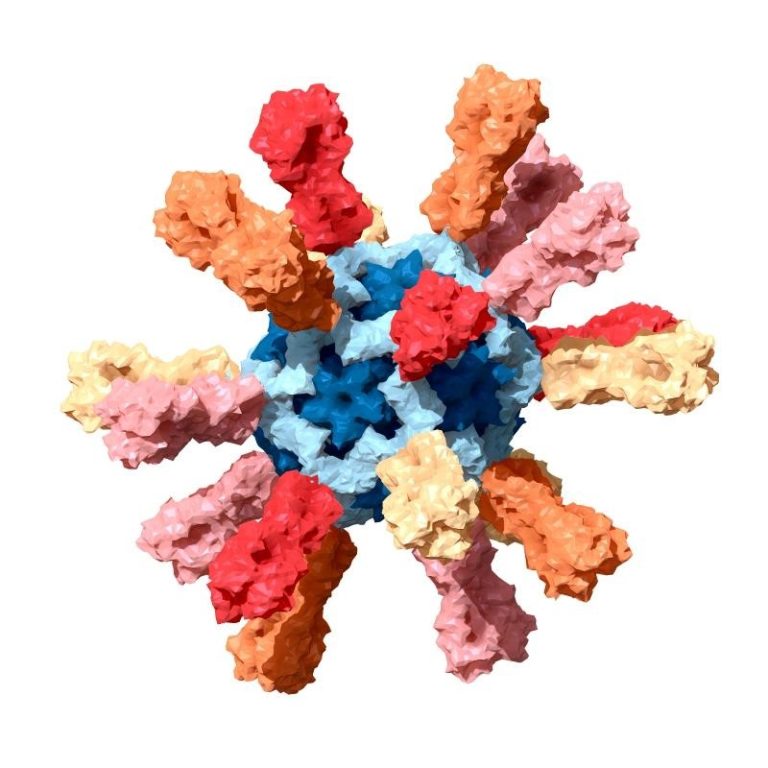
On Jun. 1, 2021, the National Institutes of Health (NIH) announced the first-in-human, Phase 1 trial assessing the…

On Apr. 9, 2021, the American Chemical Society awarded The Priestley Medal to Paul Alivisatos “to recognize distinguished…

On Jan. 28, 2021, Celsion announced the filing of a provisional U.S. patent application for a novel DNA-based,…

On Jan. 11, 2021, Baxter announced an agreement to provide sterile manufacturing services for NVX-CoV2373, Novavax COVID-19 recombinant…

On Dec. 22, 2020, the NIH researchers announced they had isolated a set of promising, tiny antibodies, or…

On Dec. 7, 2020, Matinas BioPharma announced that they planned to collaborate with the National Institute of Allergy…

On Oct. 27, 2020, Novavax announced updates on its Phase 3 clinical development program of NVX-CoV2373, its COVID-19…

On Oct. 22, 2020, Amyris and the Infectious Disease Research Institute (IDRI) announced the signing of a Collaboration…

On Oct. 19, 2020, Alberta Enterprise announced that it had invested an additional $5 million into its third…

On Sept. 25, 2020, scientists from the University of Oxfordメs Nuffield Department of Medicine published their evaluation of…

On Sept. 16, 2020, NanoViricides announced that it had nominated a clinical drug candidate for the treatment of…

On Sept. 15, 2020, Novavax announced an amendment to its existing agreement with Serum Institute of India which…

On Aug. 24, 2020, Novavax announced that the first volunteers were enrolled in the Phase 2 portion of…

On Aug. 17, 2020, Novavax announced the beginning of a Phase 2b clinical trial in South Africa to…

On Aug. 14, 2020, Novavax announced it had signed a Heads of Terms (Term Sheet) with the Government…

On Aug. 13, 2020, Novavax and SK bioscience announced a development and supply agreement for the antigen component…
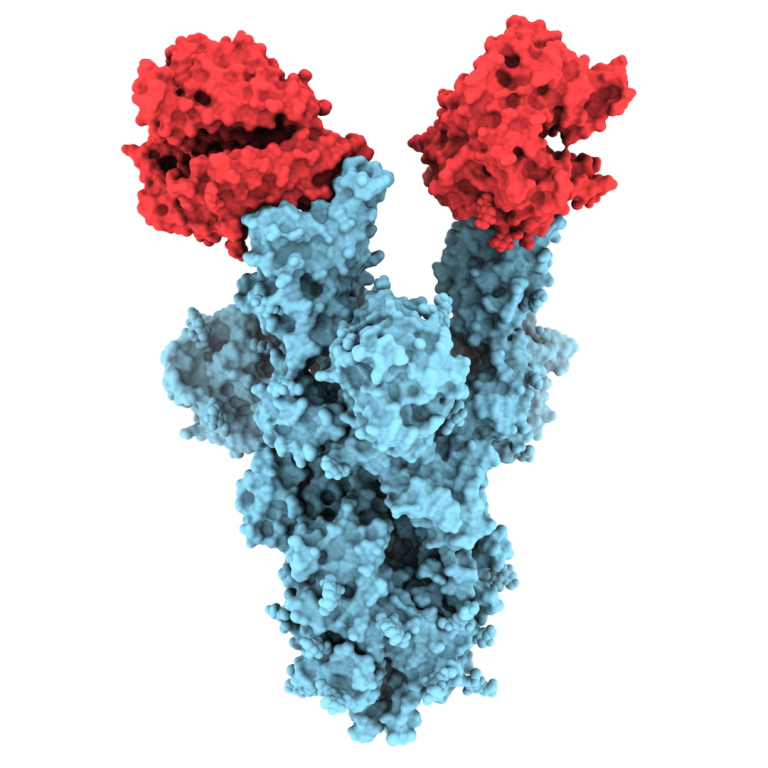
On Aug. 7, 2020, scientists at Scripps Research announced they had obtained high-resolution, atomic-scale details of the structure…

On Aug. 7, 2020, Novavax and Takeda Pharmaceutical announced a partnership for the development, manufacturing and commercialization of…

On Aug. 6, 2020, Novavax announced it had entered a supply and license agreement with the Serum Institute…

On Aug. 4, 2020, Novavax announced Phase 1 data from its Phase 1/2 randomized, observer-blinded, placebo-controlled trial of…

On Aug. 1, 2020, Novavax announced it had received approval to start human trials in India for the…

On Jul. 23, 2020, Novavax and FUJIFILM Diosynth Biotechnologies announced an agreement to manufacture bulk drug substance for…

On Jul. 20, 2020, scientists at HDT Bio, PAI Life Sciences, and the University of Washington announced they…

On Jul. 13, 2020, a team of researchers from Oxford University, the Rosalind Franklin Institute, Diamond Light Source…

On Jul. 8, 2020, NanoViricides announced that excellent safety and tolerability of the drug candidates it is developing…