UK Nice leads the way in approving breakthrough treatment for multiple myeloma
On Jun. 13, 2025, people in England will become the first in the world to receive belantamab mafodotin…
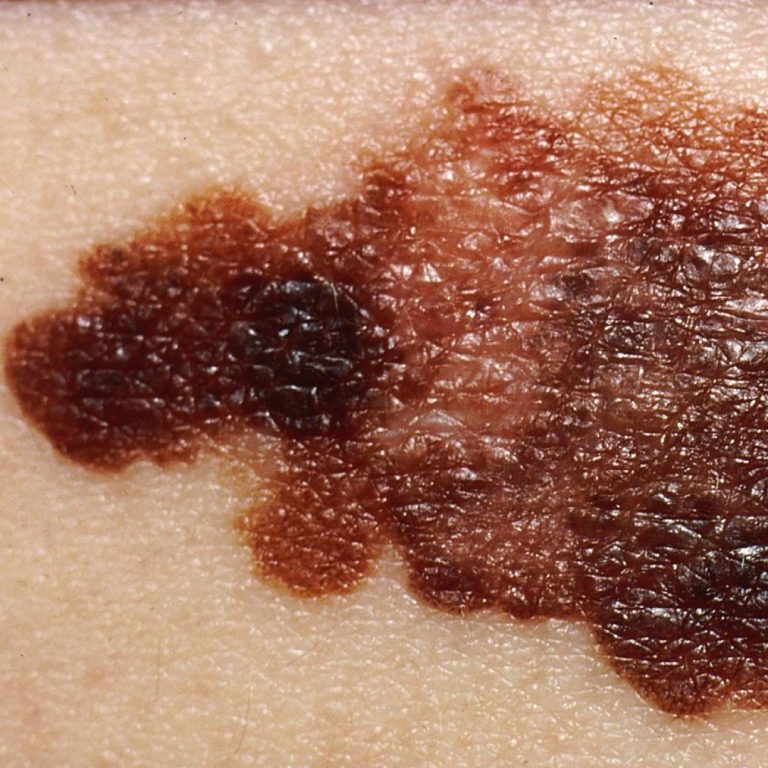
On Jun. 13, 2025, people in England will become the first in the world to receive belantamab mafodotin…

On Jun. 4, 2025, the Conference of State Parties (CoSP) of the African Medicines Agency (AMA) announced the…
On May 22, 2025, Sanofi announces the formal opening of the company’s new flagship U.S. offices, unveiling a…

On May 20, 2025, the World Health Organization (WHO) announced that world leaders pledged at least an additional…

On May 19, 2025, Prime Medicine announced positive initial data from the first patient dosed in its ongoing…

On May 9, 2025, a McGill University led team of researchers announced a study that shows an estimated…

On May 7, 2025, researchers at the University College London (UCL) and King’s College London announced that an…

On May 5, 2025, Bristol Myers Squibb said it will invest $40 billion in the U.S. over the…

On Apr. 22, 2025, Regeneron Pharmaceuticals announced a significant expansion of its manufacturing capacity through a new agreement…

On Apr. 10, 2025, Novartis, a leading global innovative medicines company, announced a planned $23 billion investment over…

On Apr. 1, 2025, layoff notices began arriving for thousands of employees of the sprawling U.S. Department of…

On Mar. 26, 2025, the Biotechnology Innovation Organization (BIO) released results from a membership survey that underscores the…

On Mar. 7, 2025, a team of researchers from Aalto University and the University of Bayreuth announced that…

On Mar. 3, 2025, LifeScienceHistory.com: Where history is made daily announces the release of a rewritten hit rock…

On Mar. 3, 2025, Genentech announced that the U.S. Food and Drug Administration (FDA) has approved TNKase® (tenecteplase),…
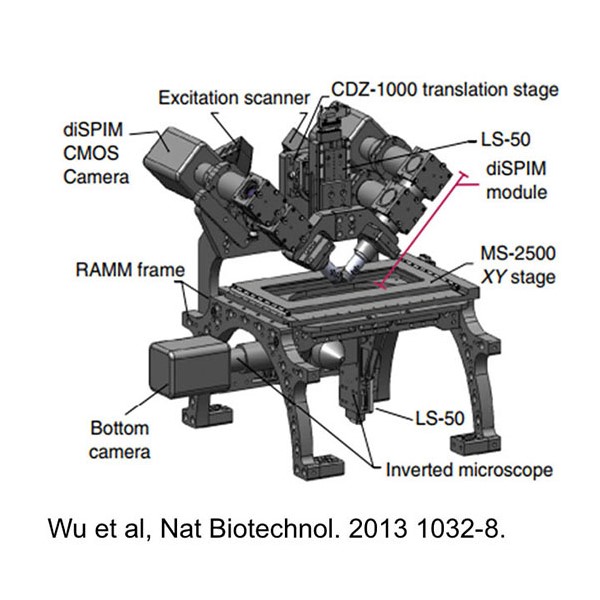
On Feb. 21, 2025, a team of researchers from the Marine Biological Laboratory (MBL) announced a hybrid custom-designed…

Jan. 24, 2025, the U.S. Food and Drug Administration (FDA) pulled draft guidance from its website requiring companies…
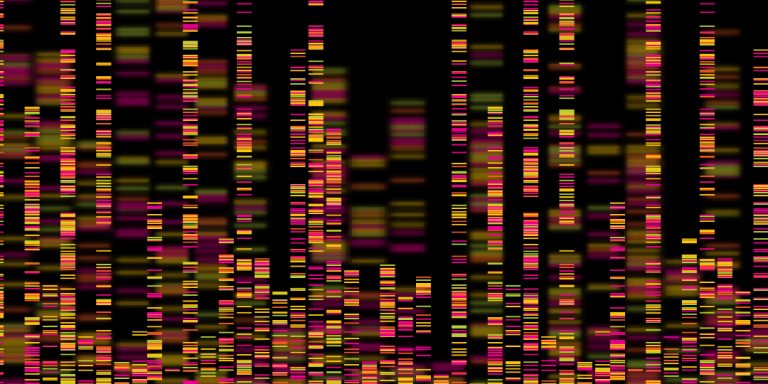
On Jan. 22, 2025, scientists at deCODE genetics/Amgen announced they have constructed a complete map of how human…
On Jan. 21, 2025, Rice University announced that four research groups are part of an inaugural cohort of…

On Jan. 14, 2025, the U.S. Food and Drug Administration’s Center for Drug Evaluation and Research (CDER) announced…

On Jan. 10, 2025, the Institute for Health Metrics and Evaluation (IHME) reported that when it comes to…

On Dec. 11, 2024, Massachusetts General Hospital (MGH), founding member of the Mass General Brigham healthcare system, announced…
On Dec. 11, 2024, the World Health Organization (WHO) reported new data has revealed that an estimated 2.2…
On Dec. 2, 2024, the University of Colorado Anschutz Medical Campus announced it will receive up to $46…

On Nov. 5, 2024, a World Health Organization (WHO) study published in eBioMedicine named 17 pathogens that regularly…

On Oct. 23, 2024, the University of Rochester announced that the Saunders Foundation, led by University Trustee Emeritus…
On Oct. 21, 2024, Rice University’s Baker Institute for Public Policy reported that hospital service prices surged more…

On Oct. 16, 2024, Sanofi announced it will contribute $18 million to three Historically Black Medical Schools to…
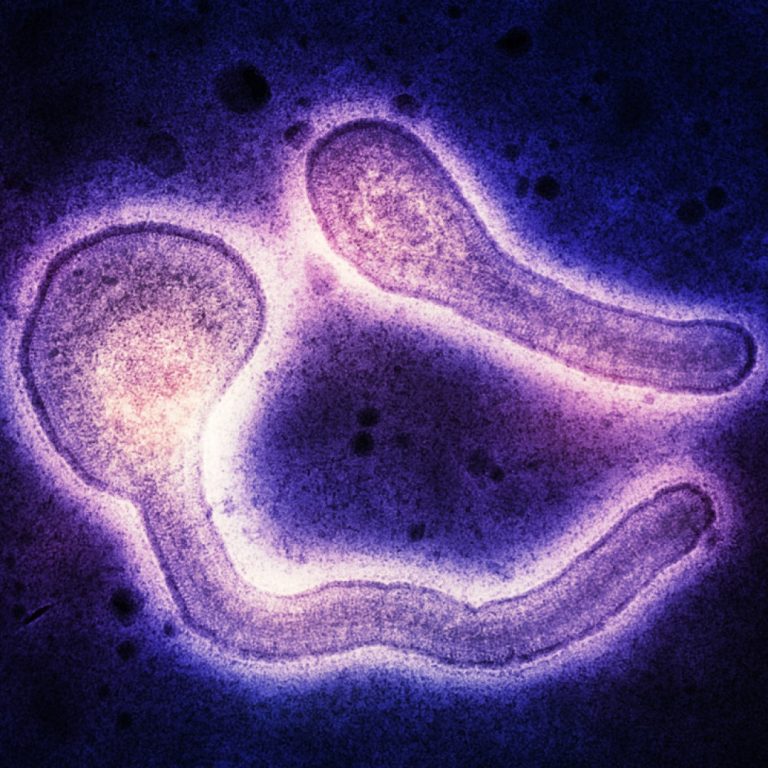
On Oct. 11, 2024, the World Health Organization (WHO) reported that a total of 58 cases of Marburg…

On Oct. 9, 2024, the Nobel Foundation awarded the Nobel Prize in Chemistry: one half to David Baker,…