FDA issued Emergency Use Authorization for Philips’ acute care patient monitoring solutions for hospitals
On Jul. 2, 2020, Royal Philips announced that the FDA had issued an Emergency Use Authorization (EUA) for…

On Jul. 2, 2020, Royal Philips announced that the FDA had issued an Emergency Use Authorization (EUA) for…

On Jul. 2, 2020, Ceres Nanosciences and its collaborators at George Mason University announced they had have developed…

On Jun. 29, 2020, the U.S. Dept. of Veterans Affairs (VA) announced it had launched a digital COVID-19…
On Jun. 29, 2020, Todos Medical announced that it had entered into a partnership with Meridian Health Services…

On Jun. 26, 2020, the COVID-19 Therapeutics Accelerator donors and partners announced the formation of the International COVID-19…

On Jun. 25, 2020, in support of Mayo Clinic’s digital health and practice transformation initiatives, the Mayo Clinic…

On Jun. 23, 2020, INOVIO announced it had received $71 million funding from the U.S. Department of Defense…

On Jun. 15, 2020, the U.S. Food and Drug Administration (FDA) permitted marketing of the first game-based digital…

On May 11, 2020, Todos Medical announced an exclusive distribution agreement with Gnomegen for the distribution of its…

On May 8, 2020, AbbVie announced it had completed its acquisition of Allergan following receipt of regulatory approval…

On May 7, 2020, Royal DSM announced it had started manufacturing nose swabs for the first time to…

On May 7, 2020, OPTI Medical Systems, an IDEXX Laboratories subsidiary announced the U.S. Food and Drug Administration…
On May 5, 2020, LabCorp announced that its COVID-19 Immunoglobulin G (IgG) antibody test is now available for…

On May 4, 2020, the U.S. Patent and Trademark Office (USPTO) unveiled a new web-based intellectual property marketplace…

On May 4, 2020, DNA Genotek, a subsidiary of OraSure Technologies, announced that its ORAcollect RNA kit (OR-100)…
On Apr. 29, 2020, Scripps researchers announced they had developed a method for examining the machinery of HIV…

On Apr. 28, 2020, the Dutch consortium of AFPRO Filters, Royal Auping, and Royal DSM began large-scale production…
On Apr. 27, 2020, Edwards Lifesciences announced that Health Canada approved an expanded indication for use of the…

On Apr. 27, 2020, iBio announced it has joined the National Institute for Innovation in Manufacturing Biopharmaceuticals (NIIMBL),…

On Apr. 24, 2020, Oregon Health & Science University (OHSU) announced that a research team led by Albert…

On Apr. 23, 2020, CHF Solutions announced that it had shipped the first commercial orders of its next…

On Apr. 23, 2020, Daxor Corp. announced that the U.S. Department of Defense (DOD) had awarded the Company…

On Apr. 22, 2020, LivaNova announced that several of its cardiopulmonary products are now permitted to be used…

On Apr. 22, 2020, the Biomedical Advanced Research and Development Authority (BARDA) and Tangen Biosciences announced teaming up…

On Apr. 22, 2020, Engineers at the McKelvey School of Engineering at Washington University in St. Louis announced…
On Apr. 22, 2020, LabCorp announced expanded serological testing for SARS-CoV-2, the virus that causes COVID-19, to more…
On Apr. 21, 2020, to fight the novel coronavirus pandemic, Cleveland Clinic and SAS announced they had created…
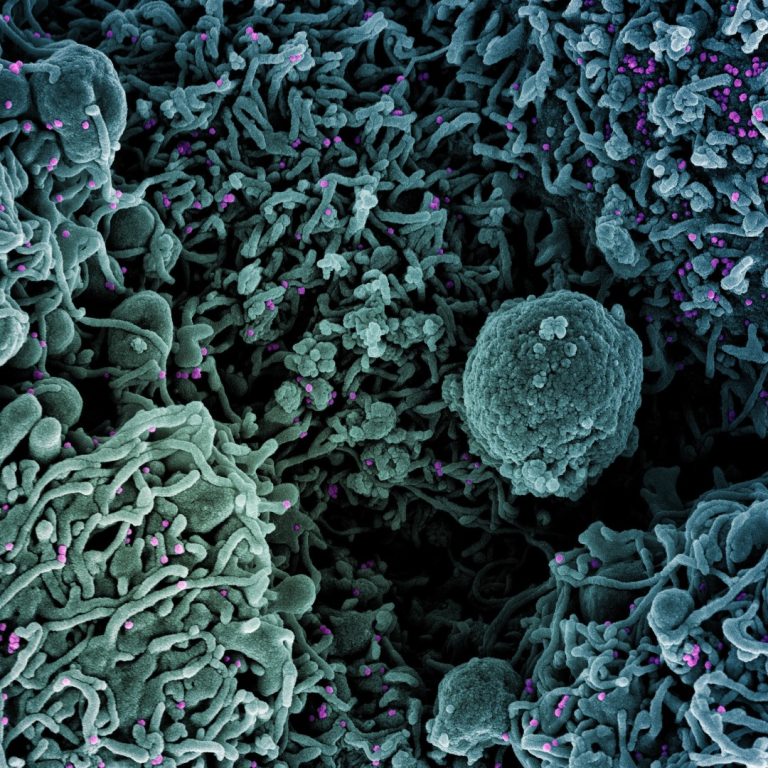
On Apr. 17, 2020, after promising COVID-19 treatments in Germany, France, Italy, Spain and the U.S,, ExThera Medical’s…

On Apr. 15, 2020, the U.S. Food and Drug Administration (FDA) authorized the production and use of a…

On Apr. 15, 2020, Todos Medical announced the FDA has accepted Todos Medical’s application for Medical Device Establishment…