Moderna advanced late-stage development of vaccine (mRNA-1273) against COVID-19
On Jun. 11, 2020, Moderna announced progress on late-stage development of mRNA-1273, the Company’s mRNA vaccine candidate against…

On Jun. 11, 2020, Moderna announced progress on late-stage development of mRNA-1273, the Company’s mRNA vaccine candidate against…
On Jun. 11, 2020, Regeneron announced that Science had accepted for publication two papers describing the creation of…
On Jun. 10, 2020, researchers funded by the National Institutes of Health (NIH) launched an effort to evaluate…

On Jun. 10, 2020, African Americans who smoke are nearly 2.5 times more likely to have a stroke…

On Jun. 10, 2020, Washington University School of Medicine in St. Louis reported they had developed a mouse…

On Jun. 10, 2020, OraSure Technologies announced it had been awarded a $629,217 contract from the Biomedical Advanced…

On Jun. 10, 2020, RedHill Biopharma announced it had submitted a Clinical Trial Application (CTA) with the Ministry…

On Jun. 9, 2020, an international gnomAD team of over 100 scientists released its first set of discoveries…
On Jun. 9, 2020, Illumina announced the U.S. Food and Drug Administration (FDA) issued an Emergency Use Authorization…

On Jun. 9, 2020, Quidel announced it had received an amended Emergency Use Authorization (EUA) from the FDA,…

On Jun. 8, 2020, researchers at the National Human Genome Research Institute announced they had discovered clues to…
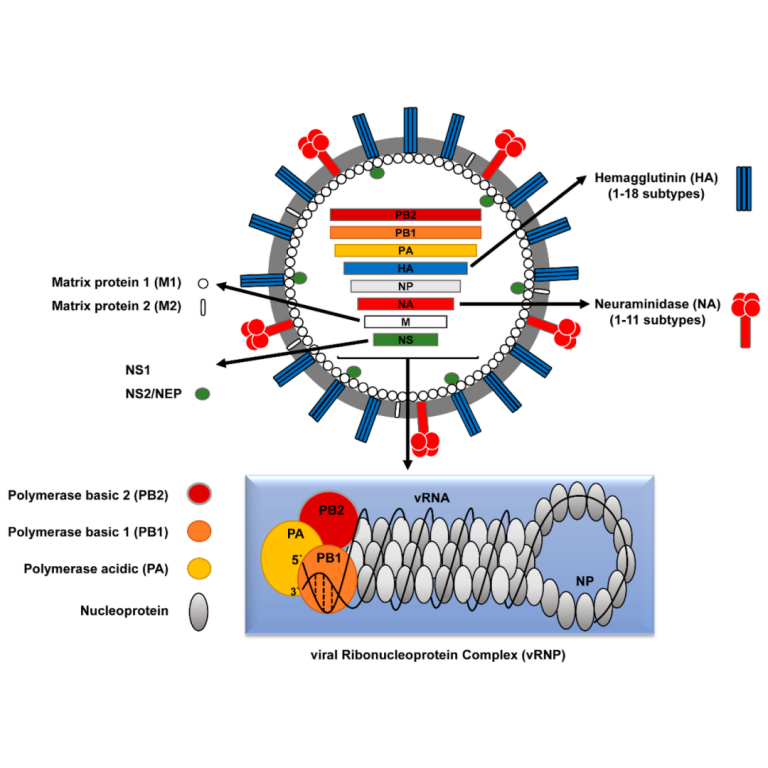
On Jun. 8, 2020, Intravacc and Versatope announced that they have signed a research service agreement to further…

On Jun. 8, 2020, Evotec announced that its Seattle-based subsidiary Just – Evotec Biologics had entered into an…
On Jun. 5, 2020, the Chief Investigators of the randomised evaluation of COVid-19 thERapY (RECOVERY) trial on hydroxychloroquine…
On Jun. 5, 2020, Sorrento announced it had completed a preclinical batch of the STI-4398 (COVIDTRAP) protein and…

On Jun. 5, 2020, CEPI, the Coalition for Epidemic Preparedness Innovations, CSL and The University of Queensland (UQ)…

On Jun. 4, 2020, world leaders pledged an additional US$ 8.8 billion for Gavi, the Vaccine Alliance, far…

On Jun. 4, 2020, the U.S. Department of Health and Human Services (HHS) announced new Guidance that specified…

On Jun. 4, 2020, the Bill & Melinda Gates Foundation announced a five-year, US$1.6 billion commitment to Gavi,…
On Jun. 4, 2020, the World Health Organization (WHO) welcomed funding commitments from Gavi, the Vaccine Alliance’s third…

On Jun. 4, 2020, Novavax announced that the company had been awarded a contract by the U.S. Department…

On Jun. 4, 2020, INOVIO and Seoul National University Hospital announced a partnership to start a Phase 1/2…
On Jun. 1, 2020, Gilead Sciences announced topline results from the Phase 3 SIMPLE trial in hospitalized patients…

On Jun. 1, 2020, Emergent BioSolutions announced it had been issued a task order under an existing contract…
On Jun. 1, 2020, Anixa Biosciences and OntoChem announced they had synthesized four potential Covid-19 compounds that will…

On Jun. 1, 2020, SIGA Technologies announced its first international delivery of TPOXX (tecovirimat), with 2,500 courses delivered…

On May 29, 2020, the World Health Organization (WHO) announced that thirty countries and multiple international partners and…

On May 29, 2020, the California Institute for Regenerative Medicine (CIRM) approved investments in three early-stage research programs…

On May 29, 2020, Moderna announced the first participants in each age cohort had been dosed in the…
On May 29, 2020, Johnson & Johnson announced that its Janssen Pharmaceutical subsidiary received a positive opinion from…