Biopharma leaders united to stand with science
On Sept. 8, 2020, the CEOs of AstraZeneca, BioNTech, GlaxoSmithKline, Johnson & Johnson, Merck, Moderna, Novavax, Pfizer, and…
On Sept. 8, 2020, the CEOs of AstraZeneca, BioNTech, GlaxoSmithKline, Johnson & Johnson, Merck, Moderna, Novavax, Pfizer, and…
On Sept. 8, 2020, RedHill Biopharma announced that opaganib demonstrated potent inhibition of SARS-CoV-2, the virus that causes…

On Sept. 8, 2020, LabCorp announced the launch of the first testing method to simultaneously detect COVID-19, influenza…

On Sept. 8, 2020, INOVIO announced that Thermo Fisher Scientific, the world leader in serving science, had signed…

On Sept. 7, 2020, research from the Children’s Medical Center Research Institute at UT Southwestern (CRI) determined how…

On Sept. 7, 2020, DiaSorin Molecular announced that it had received U.S. Food and Drug Administration (FDA) Clearance…
On Sept. 3, 2020, RedHill Biopharma announced the selection of opaganib, a proprietary, first-in-class, orally-administered, sphingosine kinase-2 (SK2)…
On Sept. 3, 2020, Humanigen announced the first case-control data of lenzilumab in severe COVID-19 demonstrated an 80%…

On Sept. 3, 2020, the U.S Department of Defense announced that five locations were identified to participate in…
On Sept. 3, 2020, Sanofi and GSK announced the start of the Phase 1/2 clinical trial for their…

On Sept. 3, 2020, BioCryst Pharmaceuticals announced that the U.S. Department of Health and Human Services (HHS) had…
On Sept. 3, 2020, Oregon Health & Science University (OHSU) announced that for the first time, early research…
On Sept. 3, 2020, OraSure Technologies announced that its ORAcollect-ᄋRNA (OR-100) collection device was included along with other…

On Sept. 3, 2020, a team of Virginia Tech researchers announced that they will begin testing wastewater at…

On Sept. 3, 2020, Roche announced that the cobas SARS-CoV-2 & Influenza A/B Test for use on the…

On Sept. 2, 2020, Mesoblast announced that it had received ethics approval to include Australian hospitals in the…

On Sept. 2, 2020, Novavax announced the publication in The New England Journal of Medicine of Phase 1…
On Sept. 2, 2020, the Janssen Pharmaceutical Companies of Johnson & Johnson announced that it had made a…

On Sept. 2, 2020, PathGroup announced the award of a funding grant from the National Institutes of Health…
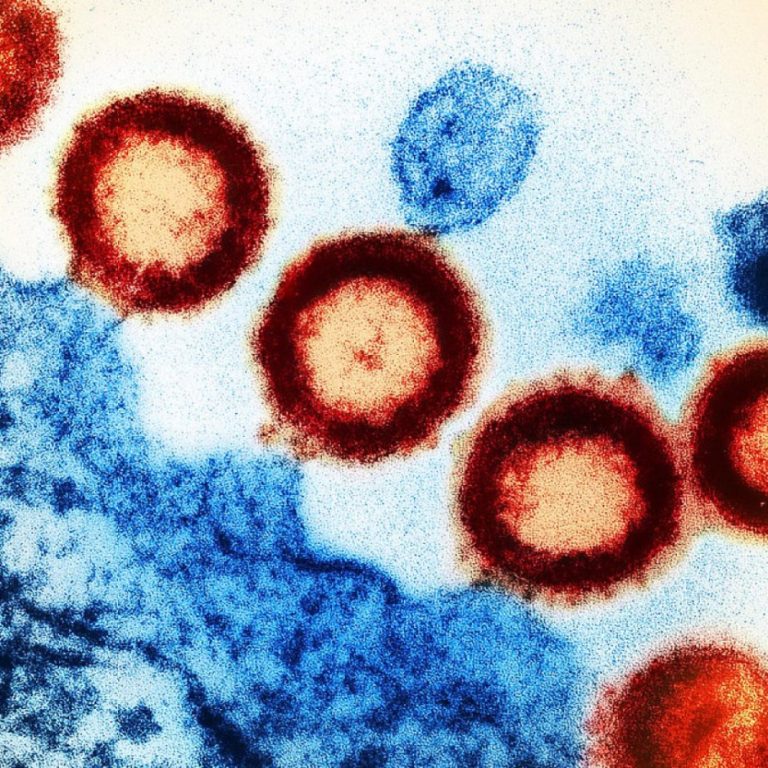
On Sept. 2, 2020, UT Southwestern announced that it had performed the first HIV-positive-to-HIV-positive organ transplant in Texas….

On Sept. 2, 2020, MicroGEM announced it had been awarded up to $40.9 million by the National Institutes…

On Sept. 1, 2020, scientists at deCODE genetics in Iceland, a subsidiary of Amgen, published a study in…

On Sept. 1, 2020, Amyris announced that it had successfully scaled up the commercial production of Cannabigerol (CBG)…
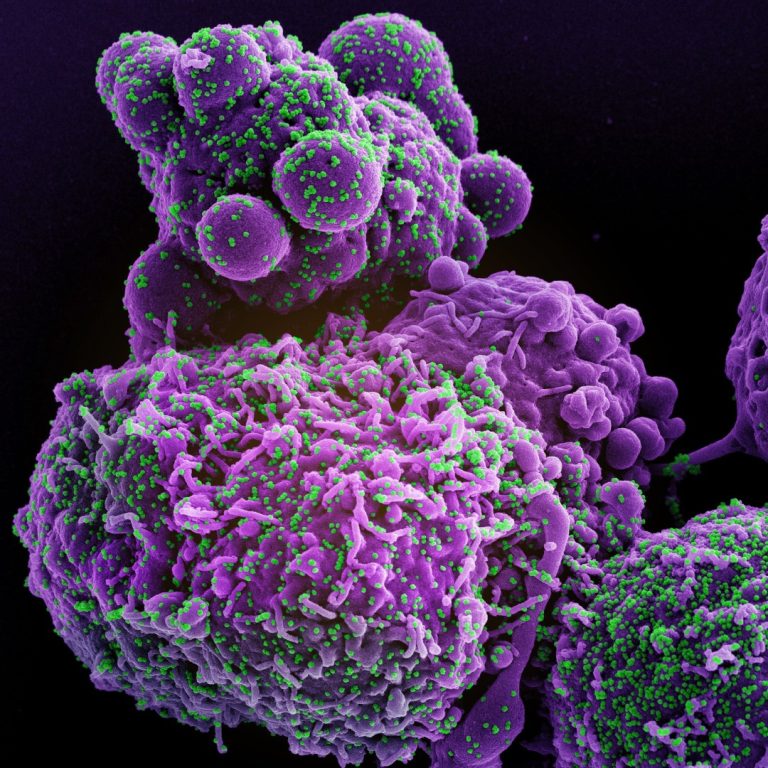
On Sept. 1, 2020, Sanofi announced that the global Phase 3 trial investigating intravenously administered Kevzara (sarilumab) at…

On Aug. 31, 2020, in a study published in PNAS, researchers used conservation biology and genomics to discover…

On Aug. 31, 2020, AstraZeneca announced that the COVID-19 vaccine AZD1222 expanded into U.S. Phase III clinical trial…
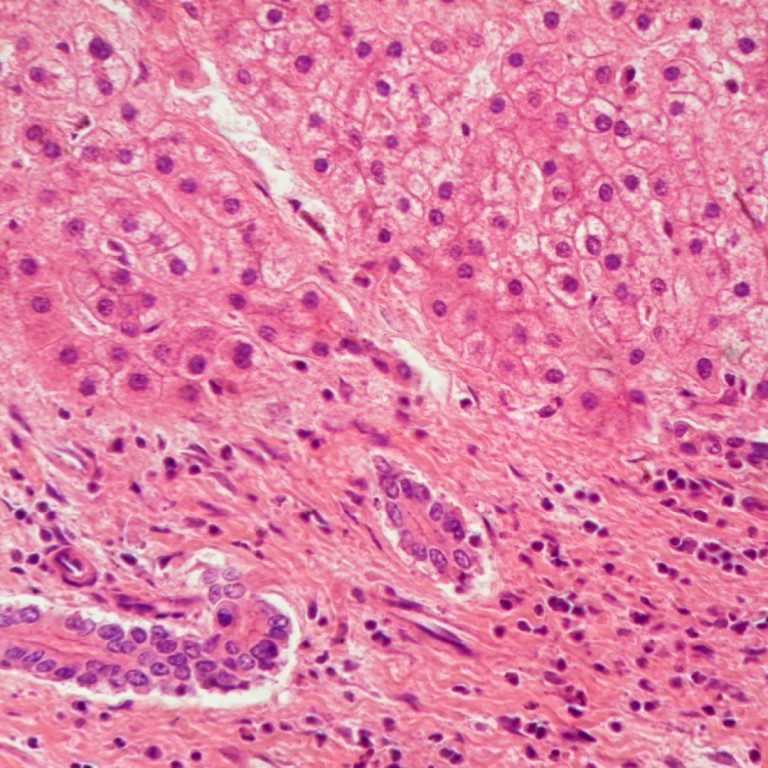
On Aug. 31, 2020, an international team of scientists supported by the National Institutes of Health (NIH) announced…

On Aug. 31, 2020, Novavax announced it had reached an agreement in principle with the Government of Canada…

On Aug. 31, 2020, BioCryst Pharmaceuticals announced that the National Institute of Allergy and Infectious Diseases (NIAID) had…

On Aug. 28, 2020, Moderna confirmed that the Company was engaged in discussions with the Ministry of Health,…