The CDC updated ‘how COVID is spread’
On Oct. 5, 2020, the CDC announced the existence of published reports that showed limited, uncommon circumstances where…

On Oct. 5, 2020, the CDC announced the existence of published reports that showed limited, uncommon circumstances where…

On Oct. 5, 2020, Mateon Therapeutics announced that ArtiShieldTM had been approved for manufacture and marketing by the…

On Oct. 5, 2020, Vaxxas announced a $22 million United States government award to support the deployment of…

On Oct. 5, 2020, Corvus Pharmaceuticals announced that it has initiated a Phase 1 study to investigate a…
On Oct. 5, 2020, the Fred Hutchinson Cancer Research Center announced the opening of the COVID-19 Clinical Research…
On Oct. 2, 2020, Yale scientists announced construction of the next-generation human brain PET scanner, the NeuroeXplorer (NX),…

On Oct. 2, 2020, the WHO and the Kingdom of Saudi Arabia signed a contribution agreement in the…
On Oct. 2, 2020, Quidel announced that it had received Emergency Use Authorization from the U.S. Food and…

On Oct. 1, 2020, Amyris announced that it has begun delivering samples to pharmaceutical companies of a sustainable…

On Oct. 1, 2020, Johnson & Johnson announced it had successfully completed its acquisition of Momenta Pharmaceuticals, a…

On Sept. 30, 2020, researchers at the Karolinska Institutet in Sweden and the Max Planck Institute for Evolutionary…
On Sept. 30, 2020, ViralClear Pharmaceuticals and Sorrento Therapeutics announced the companies were exploring the synergistic potential of…

On Sept. 30, 2020, Bio-Techne announced the opening of its approximately 61,000 square foot state-of-the-art GMP (Good Manufacturing…
On Sept. 30, 2020, Quest Diagnostics announced three different test options for healthcare providers across the U.S.to aid…
On Sept. 30, 2020, BD (Becton, Dickinson) announced its rapid, point-of-care, SARS-CoV-2 antigen test for use on the…

On Sept. 30, 2020, researchers from the Perelman School of Medicine at the University of Pennsylvania reported results…
On Sept. 29, 2020, the National Institute of Allergy and Infectious Diseases announced that a phase 1 trial…

On Sept. 29, 2020, Oxford Immunotec announced that it had received clearance from the FDA to amend the…

On Sept. 29, 2020, In a new Letter, published in PNAS, researchers at La Jolla Institute for Immunology…
On Sept. 29, 2020, Moderna announced the publication of the second interim analysis of the open-label Phase 1…

On Sept. 29, 2020, AXIM Biotechnologies announced that it had filed a provisional patent for a first-in-class face…
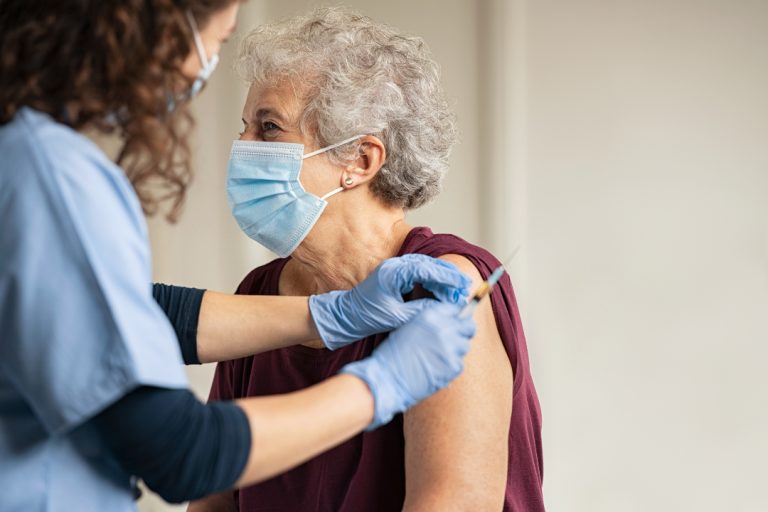
On Sept. 29, 2020, a phase 1 trial of an investigational mRNA vaccine to prevent SARS-CoV-2 infection was…
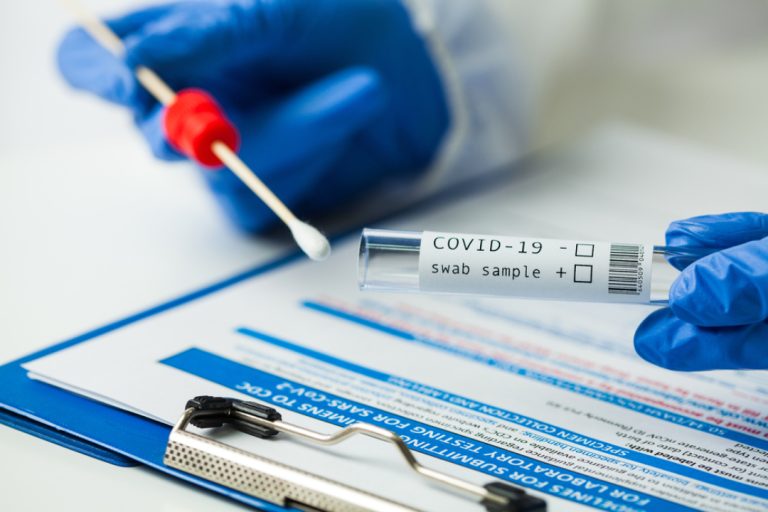
On Sept. 28, 2020, the World Health Organization announced agreements to make available, for low and middle-income countries,…

On Sept. 28, 2020, INOVIO announced the U.S. Food and Drug Administration (FDA) had notified the company that…

On Sept. 25, 2020, Todos Medical announced that it had entered into an implementation and equipment financing partnership…

On Sept. 24, 2020, an international research team led by the Max Planck Institute for Evolutionary Anthropology in…
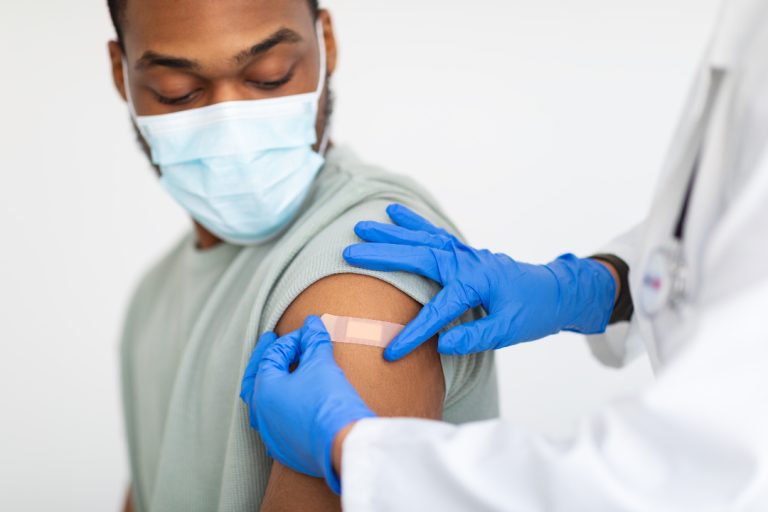
On Sept. 24, 2020, Novavax announced that it had initiated its first Phase 3 study to evaluate the…
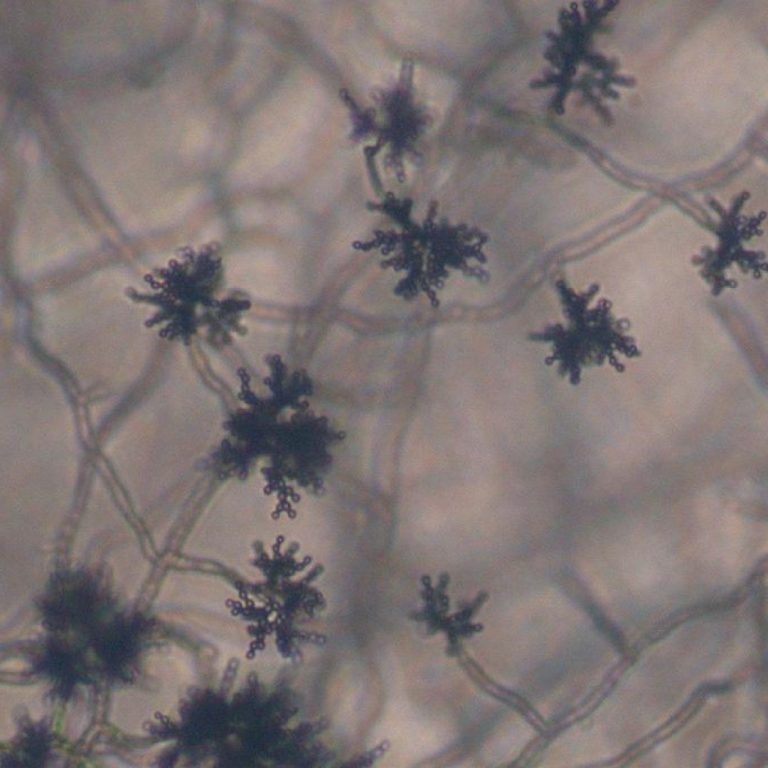
On Sept. 23, 2020, the USDA Agricultural Research Service (ARS) announced that a harmless airborne fungus, Cladosporium sphaerospermum…
On Sept. 23, 2020, Pascal Biosciences announced the Company had confirmed an earlier report that certain cannabinoids block…

On Sept. 22, 2020, RedHill Biopharma announced approval from the Brazilian Health Regulatory Agency (ANVISA) for its ongoing…