Hydroxychloroquine does not benefit adults hospitalized with COVID-19
On Nov. 9, 2020, a National Institutes of Health (NIH) clinical trial evaluating the safety and effectiveness of…
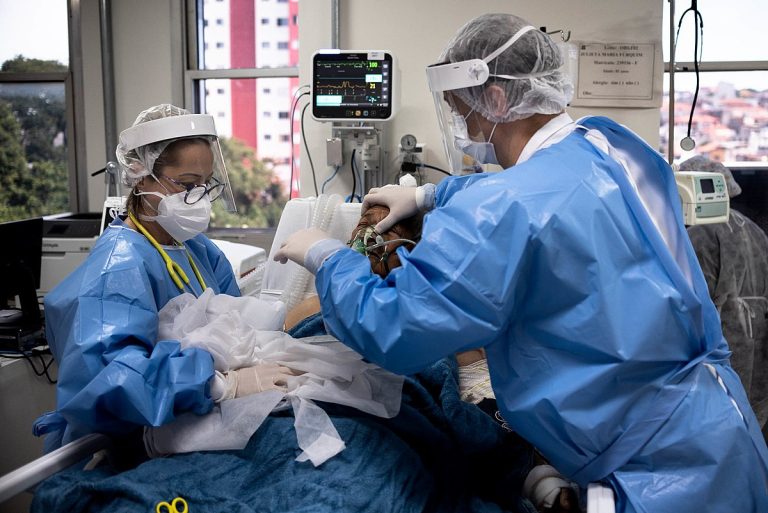
On Nov. 9, 2020, a National Institutes of Health (NIH) clinical trial evaluating the safety and effectiveness of…

On Nov. 9, 2020, a study published in JAMA reported the effect of hydroxychloroquine on clinical status at…

On Nov. 9, 2020, Ultragenyx announced that it planned to build a new large-scale gene therapy manufacturing facility…
On Nov. 9, 2020, Pfizer and BioNTech announced their mRNA-based vaccine candidate, BNT162b2, against SARS-CoV-2 had demonstrated evidence…
On Nov. 9, 2020, the Fred Hutchinson Cancer Research Center announced the start of volunteer enrollment for a…

On Nov. 8, 2020, research from the U.S. had shown that white-tailed deer were being infected with SARS-CoV-2,…
On Nov. 6, 2020, Mesa Biotech announced it had been awarded a contract up to $13 million from…

On Nov. 6, 2020, Humanigen announced that it had entered into a Cooperative Research and Development Agreement (CRADA)…

On Nov. 6, 2020, Thermo Fisher Scientific and Innoforce announced a joint venture agreement to establish a new…
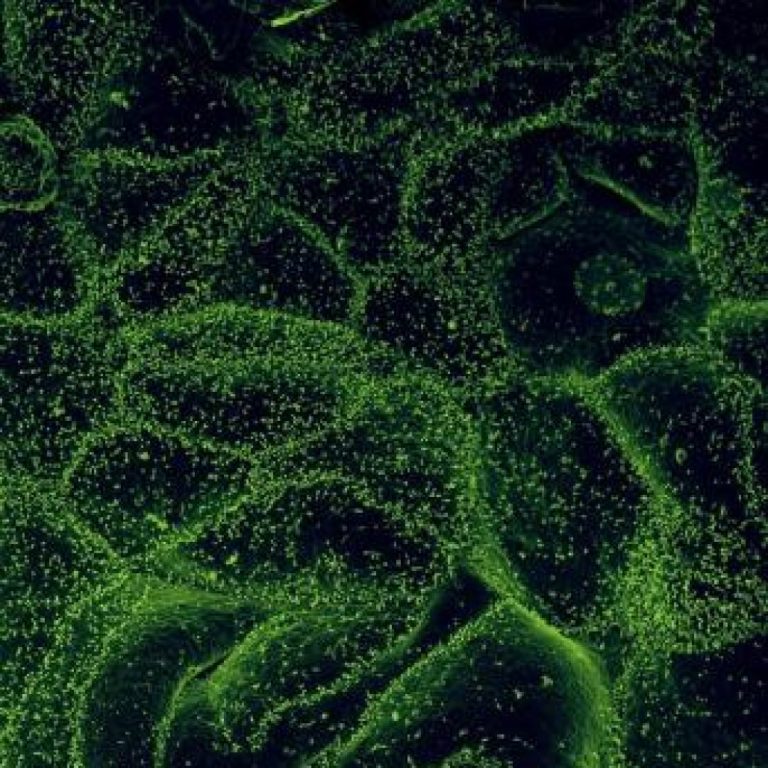
On Nov. 6, 2020, in a study published in Cell, the researchers identified antiviral defense genes that the…

On Nov. 5, 2020, Denmark announced strict lockdown rules in the north of the country after authorities discovered…

On Nov. 4, 2020, Mateon Therapeutics announced the receipt of approval from Instituto Nacional de Salud (INS), the…
On Nov. 4, 2020, Novavax announced the signing of a non-binding Heads of Terms document with the Australian…

On Nov. 4, 2020, McGill University Professor William Foulkes awarded 2020 Wilder-Penfield Prize for research in the genetics…
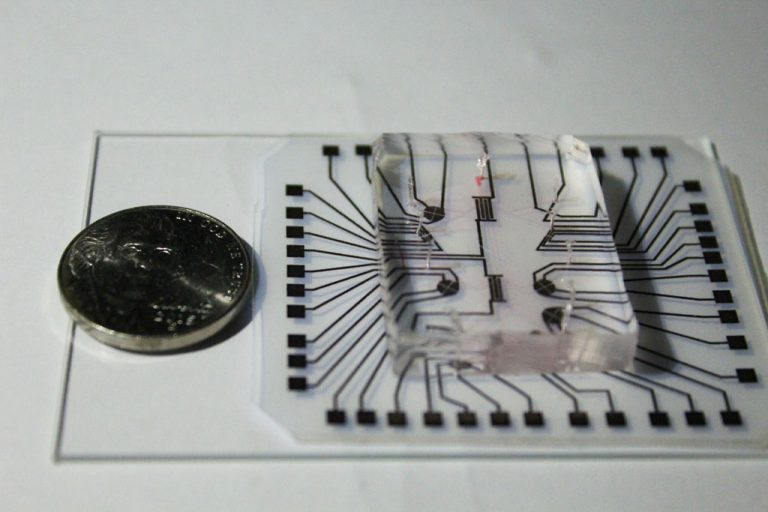
On Nov. 4, 2020, using the cutting-edge genetic editing technique known as CRISP ‘lab on a chip’ technology,…

On Nov. 3, 2020, City of Hope announced that it is investigating an innovative treatment for cancer patients…

On Nov. 3, 2020, a study published in JAMA Network Open showed that children from poorer neighborhoods performed…

On Nov. 3, 2020, Medigen Vaccine Biologics (MVC) and the National Institute of Hygiene and Epidemiology (NIHE), a…

On Nov. 3, 2020, Humanigen announced the execution of its first licensing transaction in the Asia-Pacific Region with…

On Nov. 3, 2020, OraSure Technologies announced its DNA Genotek subsidiary has received Emergency Use Authorization from the…
On Nov. 2, 2020, Novavax announced the expansion of its Maryland campus to accommodate the company’s rapid growth…

On Nov. 2, 2020, Pacific Northwest National Laboratory (PNNL) announced that in the Spring of 2020, as news…

On Nov. 1, 2020, the U.S. Department of Defense announced the start of rapid, on-site COVID-19 testing for…
On Nov. 1, 2020, scientists announced that increasing evidence shows that both natural and synthetic antimicrobial peptides (AMPs),…

On Oct. 30, 2020, Kaiser Permanente Washington Health Research Institute (KPWHRI) announced enrollment of volunteers in a phase…

On Oct. 30, 2020, the U.S. Department of Health and Human Services (HHS) and the U.S. Department of…
On Oct. 29, 2020, Mammoth Biosciences announced that it had signed agreements with MilliporeSigma and Hamilton Company targeting…

On Oct. 29, 2020, AquaBounty Technologies announced that it had identified Mayfield, Kentucky as the potential location for…

On Oct. 29, 2020, Takeda Pharmaceutical announced that it would import and distribute 50 million doses of Moderna’s…

On Oct. 29, 2020, UC Berkeley announced that it had launched a new high-throughput pop-up lab – a…