Qatar Ministry of Public Health issued Emergency Use Authorization for COVID-19 Vaccine Moderna
On Feb. 11, 2021, Moderna announced that the Qatar Ministry of Public Health had issued an emergency use…

On Feb. 11, 2021, Moderna announced that the Qatar Ministry of Public Health had issued an emergency use…

On Feb. 11, 2021, Moderna announced that the U.S. government had purchased an additional 100 million doses of…

On Feb. 11, 2021, the Oregon Department of Agriculture (ODA) lifted the quarantine on the Oregon mink farm…

On Feb. 10, 2021, researchers supported by the National Heart, Lung, and Blood Institute announced the publication of…

On Feb. 10, 2021, the United States Department of Agriculture (USDA) National Veterinary Services Laboratories announced confirmation of…
On Feb. 10, 2021, Notch Therapeutics closed $85 Million Series A financing to develop pipeline of renewable stem…

On Feb. 9, 2021, Moderna announced two supply agreements for the COVID-19 Vaccine Moderna: one with the government…
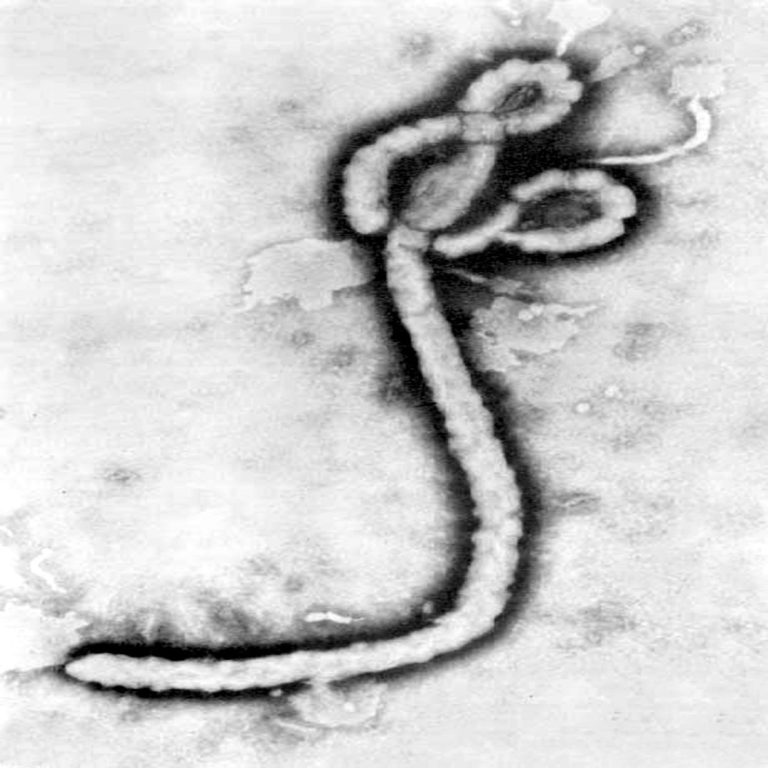
On Feb. 7, 2021, The Ministry of Health of the Democratic Republic of the Congo (DRC) announced that…

On Feb. 5, 2021, the FDA approved Breyanzi (lisocabtagene maraleucel), a cell-based gene therapy to treat adult patients…

On Feb. 4, 2021, Merck affirmed its position regarding use of ivermectin during the COVID-19 pandemic, and do…
On Feb. 4, 2021, T2 Biosystems announced that its T2SARS-CoV-2 Panel – a molecular diagnostic test that detects…

On Feb. 4, 2021, Corvus Pharmaceuticals announced that it had initiated a Phase 3 clinical trial of CPI-006…

On Feb. 4, 2021, Novavax announced the start of the rolling review process for authorization of NVX-CoV2373, its…
On Feb. 3, 2021, AXIM Biotechnologies announced initiation of clinical trials for ImmunoPass, the Companyメs rapid point-of-care test…

On Feb. 3, 2021, Quidel announced the opening of a new manufacturing facility in Carlsbad, CA that was…
On Feb. 3, 2021, IAVI and Scripps Research announced a phase 1 clinical trial testing a novel vaccine…

On Feb. 3, 2021, Moderna announced the Swiss Federal Government had increased its confirmed order commitment from 7.5…

On Feb. 3, 2021, Moderna announced that the Singapore Health Sciences Authority (HSA) had approved the interim authorization…

On Feb. 3, 2021, Novavax announced that the company had executed a binding Heads of Terms agreement with…
On Feb. 3, 2021, an experimental single-dose, intranasal influenza vaccine, was safe and produced a durable immune response…

On Feb. 3, 2021, RELIEF THERAPEUTICS affirmed that its collaboration partner NeuroRx had initiated a phase 2/3 clinical…

On Feb. 2, 2021, Mateon Therapeutics announced that its ARTI-19 trial, evaluating ARTIVeda / PulmoHeal against COVID-19 in…

On Feb. 2, 2021, Novavax announced a memorandum of understanding (MOU) with the Canadian government to produce NVX-CoV2373,…
On Jan. 31, 2021, Sorrento Therapeutics announced additional positive results from its Phase 1b study of human allogeneic…

On Jan. 28, 2021, National Institutes of Health (NIH) researchers reported that pregnant women who experienced severe symptoms…

On Jan. 28, 2021, scientists at the National Eye Institute (NEI) have developed a promising gene therapy strategy…
On Jan. 28, 2021, The WHO announced that COVID-19 cases and deaths were surging in Africa as more…

On Jan 28, 2021, Mammoth Biosciences announced a co-marketing agreement with Agilent Technologies to support the anticipated launch…
On Jan. 28, 2021, Precipio announced that following receipt of an Emergency Use Authorization from the U.S. Food…

On Jan. 28, 2021, Novavax announced that NVX-CoV2373, its protein-based COVID-19 vaccine candidate, had met the primary endpoint,…