Luminex received FDA Emergency Use Authorization and CE Mark for expanded NxTAG panel test Including SARS-CoV-2
On Mar. 4, 2021, Luminex announced that it had received Emergency Use Authorization (EUA) from the U.S. Food…

On Mar. 4, 2021, Luminex announced that it had received Emergency Use Authorization (EUA) from the U.S. Food…

On Mar. 3, 2021, Resverlogix and QIMR Berghofer Medical Research Institute announced the publishing of an article providing…

On Mar. 3, 2021, Pfizer and BioNTech, in partnership with UNICEF, announced the arrival of the first doses…
On Mar. 3, 2021, Altasciences announced support for ReAlta Life Sciences’ Phase I trial to evaluate RLS-0071 for…

On Mar. 2, 2021, Merck announced multiple agreements to support efforts to expand manufacturing capacity and supply of…
On Mar. 1, 2021, the WHO announced that the first COVID-19 vaccination campaigns in Africa using COVAX doses…

On Mar. 1, 2021, Colombia became the first country in the Americas to receive COVID-19 vaccines through the…

On Mar. 1, 2021, Quidel announced that it had received an Emergency Use Authorization (EUA) from the FDA,…
On Mar. 1, 2021, a study from Washington University School of Medicine in St. Louis provided evidence that…

On Feb. 26, 2021, Novavax and Takeda Pharmaceutical announced an exclusive license agreement for Takeda’s development, manufacturing and…

On Feb. 25, 2021, the FDA granted approval to Sarepta Therapeutics for Amondys 45 (casimersen) injection for the…

On Feb. 24, 2021, NeuroRx, announced that the phase 2b/3 trial of ZYESAMI for the treatment of Respiratory…

On Feb. 22, 2021, the World Health Organization (WHO) and Chubb signed an agreement on behalf of the…

On Feb. 22, 2021, Novavax announced it had completed enrollment of PREVENT-19, its pivotal Phase 3 study in…

On Feb. 20, 2021, Russian authorities reported the detection of influenza A(H5N8) virus infection in seven poultry workers…

On Feb. 19, 2021, BioNTech announced the submission of new data to the FDA demonstrating the stability of…

On Feb. 19, 2021, Luminex announced that it had received $11.3 million in funding from the Biomedical Advanced…

On Feb. 18, 2021, Novavax and announced a Memorandum of Understanding (MOU) with Gavi, the Vaccine Alliance, to…
On Feb. 17, 2021, Novartis announced that it had entered into a grant agreement with the Bill &…
On Feb. 17, 2021, Moderna announced that the European Commission had purchased an additional 150 million doses of…

On Feb. 17, 2021, BioNTech announced an agreement with the European Commission (EC) to supply an additional 200…
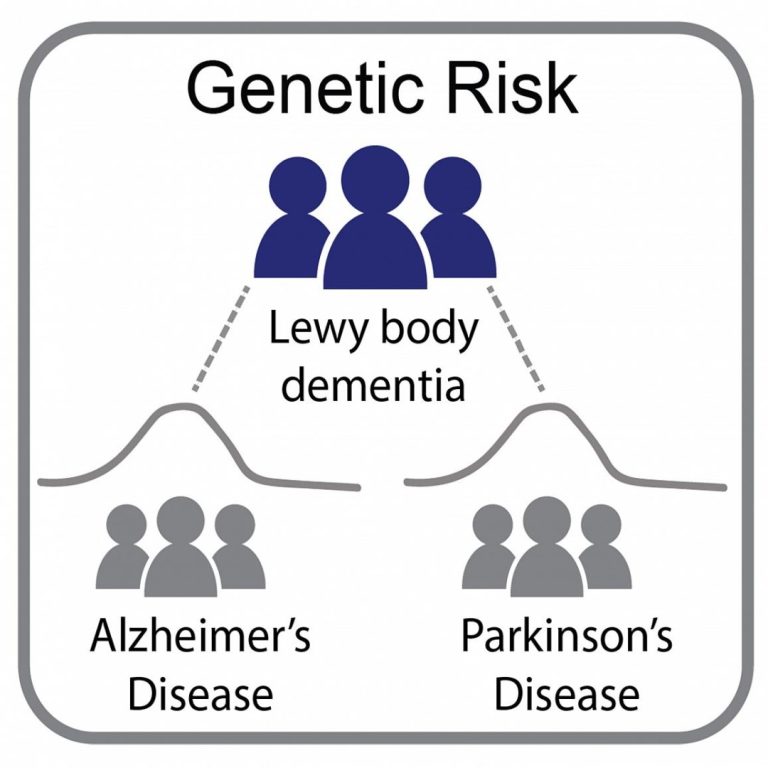
On Feb. 16, 2021, in a study led by National Institutes of Health (NIH) researchers, scientists found that…

On Feb. 16, 2021, Anixa Biosciences announced that animal testing had commenced with two proprietary compounds that have…

On Feb. 16, 2021, Moderna provided a supply update for their COVID-19 Vaccine in the United States, reporting…

On Feb. 16, 2021, Lonza, formerly Bend Research, announced that it was expanding and refining first-in-human services at…

On Feb. 15, 2021, Novavax and SK Bioscience announced an expanded collaboration and license agreement. In addition to…

On Feb. 14, 2021, the WHO announced that Health authorities in Guinea had declared an outbreak of Ebola…

On Feb. 12, 2021, Moderna announced that the Canadian Government had increased its confirmed order commitment by 4…

On Feb. 12, 2021, BD (Becton, Dickinson) announced that the U.S. Food and Drug Administration (FDA) had granted…
On Feb. 11, 2021, Cue Health announced the results of an independent clinical validation study conducted by Mayo…