Japan’s Ministry of Health, Labour and Welfare approved Moderna’s COVID-19 vaccine
On May 21, 2021, Moderna announced that the Ministry of Health, Labour and Welfare (MHLW) of Japan granted…

On May 21, 2021, Moderna announced that the Ministry of Health, Labour and Welfare (MHLW) of Japan granted…

On May 21, 2021, Moderna announced that the Ministry of Food and Drug Safety of South Korea (MFDS)…

On May 21, 2021, Novavax announced that the full results from the Phase 3, randomized, observer-blinded, placebo-controlled trial…

On May 21, 2021, Novavax announced participation in a mix-and-match (vaccine interchangeability) COVID-19 vaccine booster trial that led…
On May 20, 2021, Pfizer and BioNTech announced a new agreement with the European Commission (EC) to supply…

On May 19, 2021, Megan A. Cooper, MD, PhD, an associate professor of pediatrics at Washington University School…

On May 13, 2021, CureVac announced together with its collaboration partner GSK, first preclinical data in a rat…
On May 12, 2021, Moderna announced a new supply agreement with the government of Australia for 25 million…

On May 12, 2021, InBios announced that it had received Emergency Use Authorization from the U.S. Food and…

On May 12, 2021, InBios announced that it had received Emergency Use Authorization from the U.S. Food and…

On May 10, 2021, the National Institutes of Health reported that an investigational gene therapy can safely restore…

On May 10, 2021, AquaBounty Technologies announced that purchase orders had been received for the planned harvest of…
On May 10, 2021, Novavax announced data from a preclinical study of the company’s combination quadrivalent seasonal flu…

On May 10, 2021, Inovio Pharma announced positive safety, tolerability and immunogenicity data from its placebo-controlled and blinded…

On May 7, 2021, the World Health Organization (WHO) listed the Sinopharm COVID-19 vaccine for emergency use, giving…

On May 7, 2021, Pfizer and BioNTech announced the initiation of a Biologics License Application (BLA) with the…

On May 6, 2021, Moderna announced a new supply agreement with the Swiss Federal Government for 7 million…

On May 6, 2021, Novavax announced that it had finalized an advance purchase agreement (APA) with Gavi, the…

On May 5, 2021, researchers at Tufts University School of Engineering announced they had developed an alternative to…
On May 5, 2021, Moderna announced initial data from its Phase 2 study showing that a single 50…

On May 5, 2021, Novavax announced the publication of results from the initial primary analysis of a Phase…

On May 5, 2021, Pfizer and BioNTech announced that Health Canada had expanded the Interim Order authorization for…

On May 5, 2021, Hoth Therapeutics announced it has expanded its sponsored research agreement with Virginia Commonwealth University…

On May 5, 2021, CytoDyn announced an agreement to partner with the Albert Einstein Israelite Hospital (AEIH) in…

On May 4, 2021, Moderna announced an expansion of the Moderna Technology Center (MTC) in Norwood, MA including…
On May 3, 2021, the World Health Organization (WHO) announced the end of the 12th outbreak of Ebola…

On May 3, 2021, the World Health Organization (WHO) welcomed the Government of Swedenメs announcement to share 1…
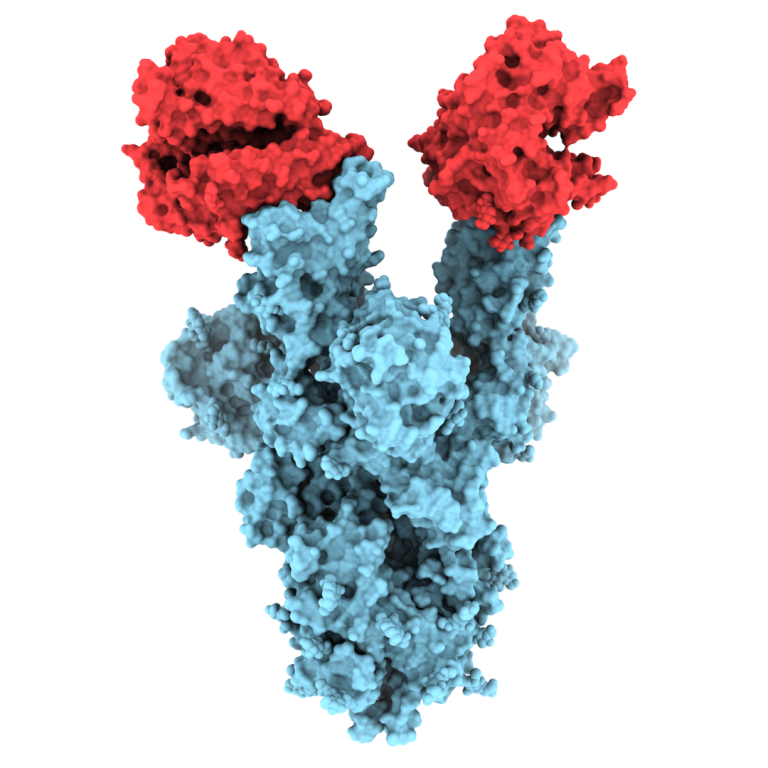
On May 3, 2021, University of British Columbia (UBC) researchers were the first in the world to publish…

On May 3, 2021, Precipio announced that it had successfully launched its COVID-19 rapid antibody test (20 minute)…

On May 3, 2021, Moderna announced an agreement with Gavi, the Vaccine Alliance to supply up to 500…