Moderna announced EMA’s CHMP recommended booster dose of Moderna’s COVID-19 vaccine in the European Union
On Oct. 25, 2021, Moderna announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human…

On Oct. 25, 2021, Moderna announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human…

On Oct. 25, 2021, scientists at Oxford University reported that Infection with COVID-19 carried a much higher risk…

On Oct. 22, 2021, the U.S. Department of Veterans Affairs (VA) announced that it had begun offering Moderna…

On Oct. 22, 2021, the U.S. Centers for Disease Control and Prevention (CDC) reported two new U.S. human…

On Oct. 21, 2021, Pfizer and BioNTech announced topline results from a Phase 3 randomized, controlled trial evaluating…

On Oct. 20, 2021, Moderna announced that the U.S. Food and Drug Administration (FDA) had authorized for emergency…
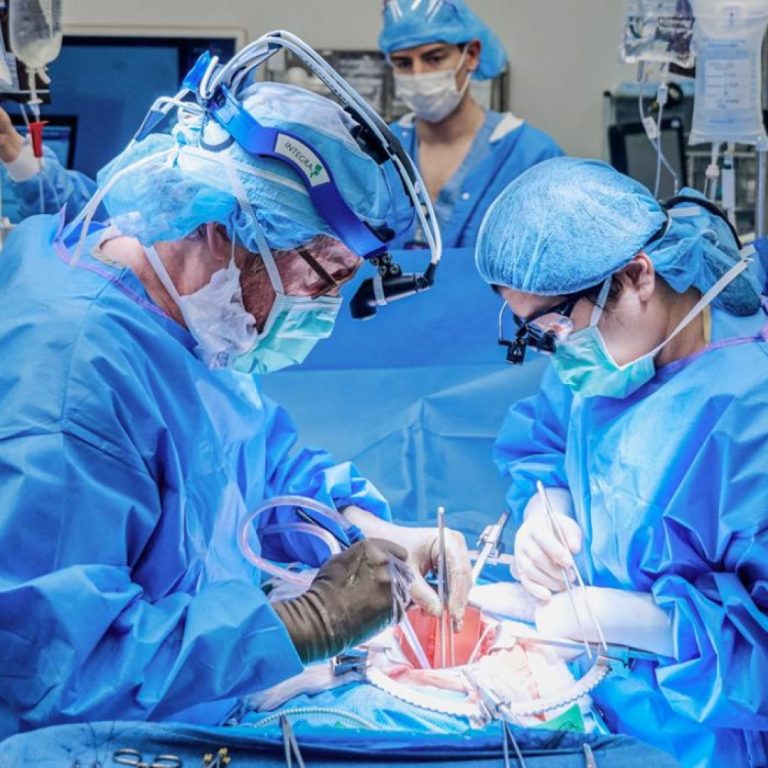
On Oct. 19, 2021, the first investigational transplant of a genetically engineered, nonhuman kidney to a human body…

On Oct. 18, 2021, Gilead Sciences announced that the Food and Drug Administration had approved a new low-dose…

On Oct. 18, 2021, Athenex and the Center for Cell and Gene Therapy at Baylor College of Medicine,…
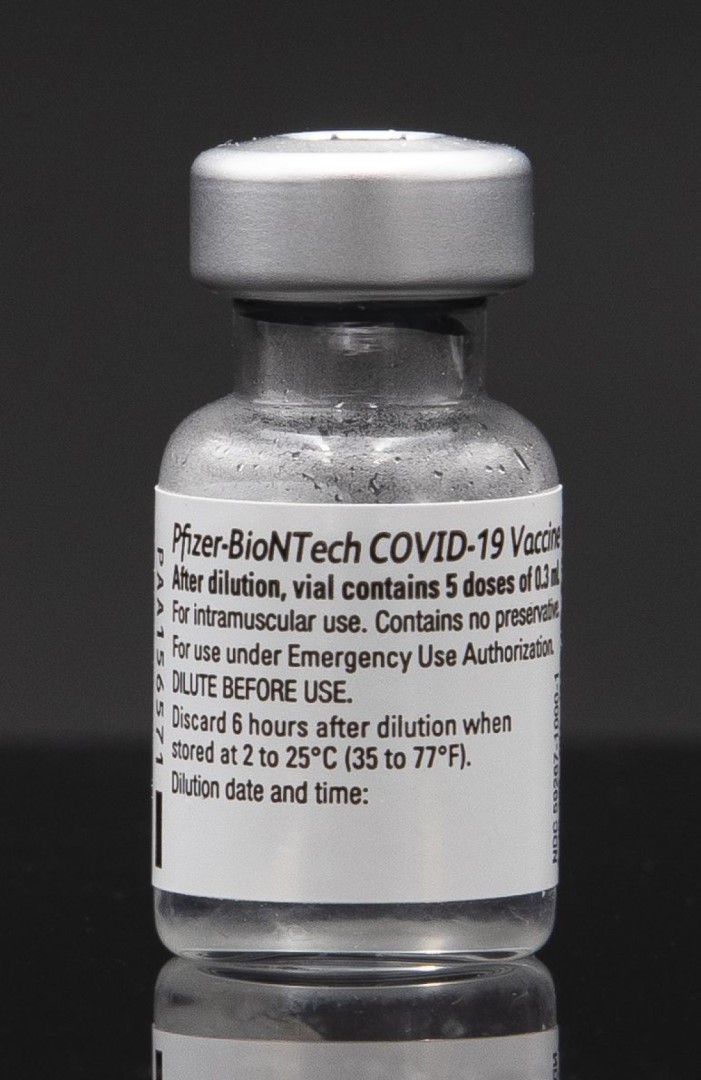
On Oct. 18, 2021, Pfizer and BioNTech announced that the European Medicines Agency’s Committee for Human Medicinal Products…

On Oct. 15, 2021, Roche announced that the U.S. Food and Drug Administration (FDA) had approved Tecentriq (atezolizumab)…
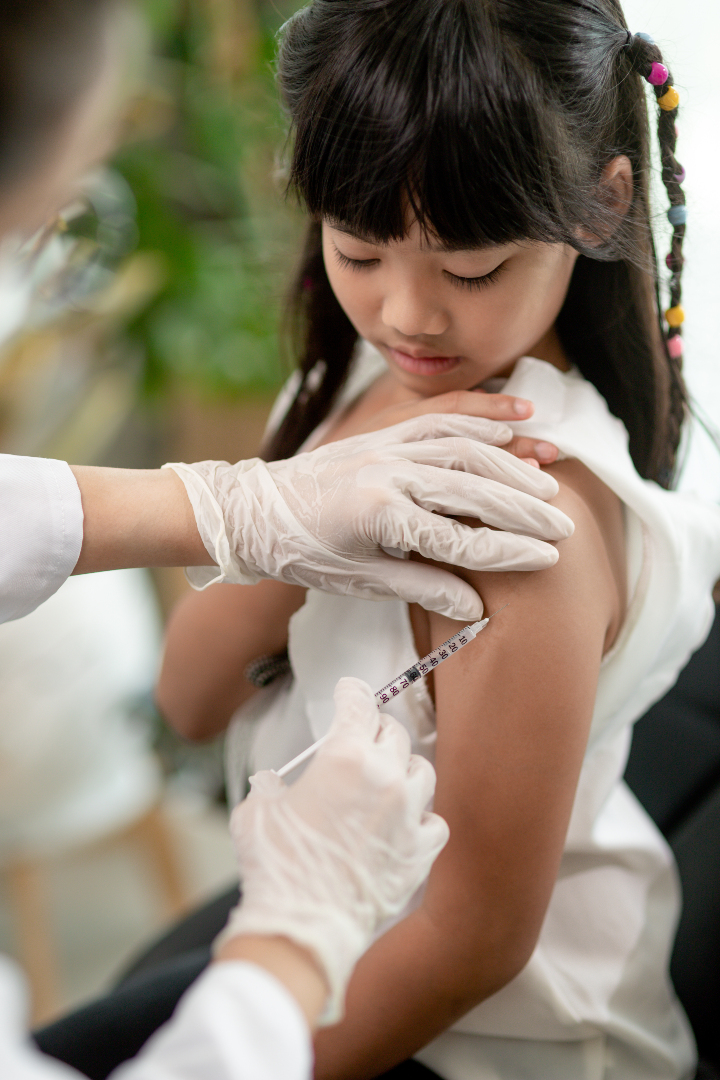
On Oct. 15, 2021, Pfizer and BioNTech announced they had submitted data supporting the vaccination of children 5…
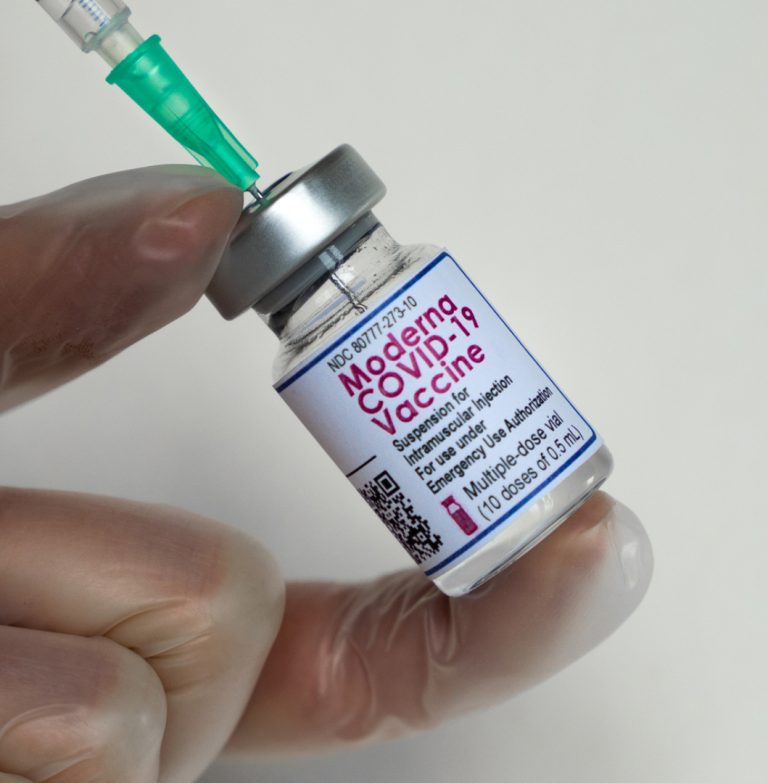
On Oct. 14, 2021, Moderna confirmed that the U.S. Food and Drug Administration’s (FDA) Vaccines and Related Biological…

On Oct. 12, 2021, Lucira Health announced that it had received PSAR approval for its Lucra CHECK IT…

On Oct. 12, 2021, Moderna announced that Gavi, the Vaccine Alliance had exercised its option to purchase an…
On Oct. 12, 2021, Inovio Pharmaceuticals announced online preprint publication in MedRxiv of Phase 1 clinical data on…

On Oct. 12, 2021, National Resilience announced a strategic collaboration with Children’s Hospital of Philadelphia (CHOP) to implement…

On Oct. 11, 2021, scientists at Washington University School of Medicine in St. Louis announced they had developed…

On Oct. 11, 2021, Inovio Pharmaceuticals announced that it had received authorization from Colombia’s INVIMA, to conduct the…

On Oct. 8, 2021, the Ministry of Health of the Democratic Republic of the Congo announced that a…
On Oct. 7, 2021, PerkinElmer announced that the U.S. Food and Drug Administration (FDA) had issued Emergency Use…

On Oct. 7, 2021, Vaxart announced that a Duke University-led study published in bioRxiv showed that Vaxart’s (investigational…
On Oct. 7, 2021, Moderna announced it was building a state-of-the-art mRNA facility in Africa with the goal…

On Oct. 7, 2021, BD (Becton, Dickinson) announced it had strengthened the U.S. government’s access to safety injection…
On Oct. 6, 2021, Washington University School of Medicine announced the start of a pediatric COVID-19 vaccine clinical…

On Oct. 5, 2021, PerkinElmer announced that the U.S. Food and Drug Administration had provided Emergency Use Authorization…

On Oct. 5, 2021, Moderna announced that the European Medicines Agency (EMA) had authorized a third dose of…

On Oct. 4, 2021, the Fred & Pamela Buffett Cancer Center announced it had successfully renewed its National…

On Oct. 4, 2021, the Nobel Prize in Physiology or Medicine was awarded to David Julius and Ardem…

On Oct. 4, 2021, Dynavax Technologies and the U.S. Department of Defense (DOD) announced Dynavax had executed an…