World Health Organization granted scond Emergency Use listing for Novavax COVID-19 vaccine
On Dec. 20, 2021, Novavax announced that the World Health Organization (WHO) had granted a second Emergency Use…

On Dec. 20, 2021, Novavax announced that the World Health Organization (WHO) had granted a second Emergency Use…

On Dec. 20, 2021, Novavax announced that the European Commission (EC) had granted Novavax conditional marketing authorization (CMA)…

On Dec. 20, 2021, Pfizer and BioNTech announced an agreement had been reached with the European Commission (EC)…
On Dec. 20, 2021, Moderna announced preliminary neutralizing antibody data against the Omicron variant following the Company’s booster…

On Dec. 17, 2021, Novavax and SK bioscience announced that the World Health Organization (WHO) had granted Emergency…
On Dec. 16, 2021, the Ebola outbreak that erupted in the Democratic Republic of the Congo’s North Kivu…

On Dec. 15, 2021, Novavax announced the submission of a New Drug Application to the Ministry of Health,…

On Dec. 14, 2021, Pfizer announced final results from an analysis of all 2,246 adults enrolled in its…
On Dec. 13, 2021, Moderna announced an agreement with the Australian Government to build a state-of-the-art messenger RNA…

On Dec. 13, 2021, Novavax announced that it had submitted a regulatory filing to the Ministry of Health…

On Dec. 10, 2021, Moderna announced an amendment to its existing contract with Gavi, the Vaccine Alliance, to…

On Dec. 10, 2021, the World Health Organization announced interim recommendations for the use of the Janssen Ad26.COV2.S…

On Dec. 9, 2021, CytoDyn announced that it had submitted a Phase 3, randomized, double blind, placebo controlled…

On Dec. 9, 2021, Moderna announced the launch of its Artificial Intelligence Academy, an innovative initiative that will…

On Dec. 8, 2021, Pfizer and BioNTech announced results from an initial laboratory study demonstrating that serum antibodies…

On Dec. 8, 2021, in a large-scale study of people from diverse ancestries, researchers narrowed down the number…

On Dec. 7, 2021, Rockefeller University scientists announced a study had demonstrated the therapeutic potential of an unusual…

On Dec. 5, 2021, Sorrento Therapeutics announced the peer-reviewed publication of a series of novel SARS-CoV-2 MPro inhibitors…

On Dec. 5, 2021, Roche announced that its planned to launch the SARS-CoV-2 & Flu A/B Rapid Antigen…
On Dec. 1, 2021, T2 Biosystems announced that its T2SARS-CoV-2 Panel detected the Omicron COVID-19 variant (B.1.1.529). To…

On Dec. 1, 2021, Moderna announced a revised supply agreement with the UK government for up to 60…
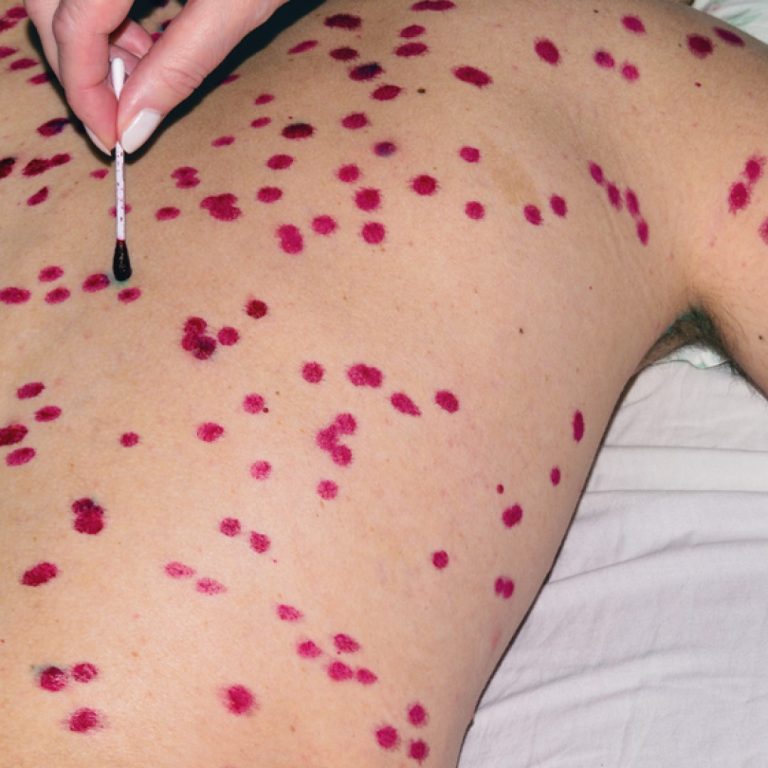
On Dec. 1, 2021, SIGA Technologies announced that Health Canada had approved oral TPOXX (tecovirimat) as an extraordinary…
On Dec. 1, 2021, the California and San Francisco Departments of Public Health confirmed that a recent case…
On Nov. 30, 2021, Inovio Pharma announced the company was rapidly moving to evaluate its COVID-19 DNA vaccine…

On Nov. 26, 2021, Merck provided an update on the MOVe-OUT study of molnupiravir, an investigational oral antiviral…

On Nov. 24, 2021, Novavax announced its submission to the Singapore Health Sciences Authority for interim authorization of…

On Nov. 24, 2021, Johnson & Johnson announced the U.S. Food and Drug Administration (FDA) had issued Emergency…

On Nov. 19, 2021, the U.S. Food and Drug Administration (FDA) announced that it had extended the emergency…
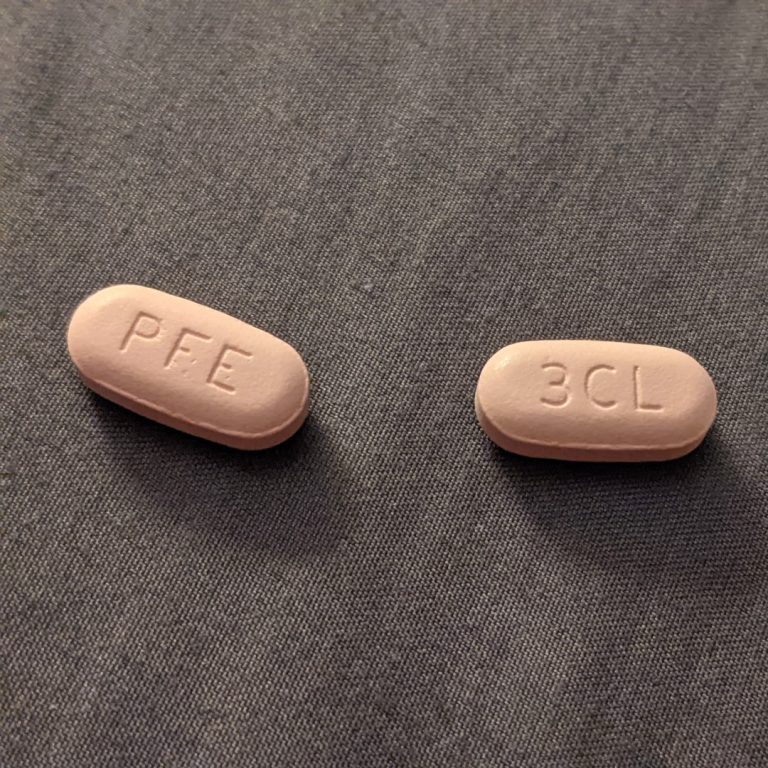
On Nov. 18, 2021, Pfizer announced an agreement with the U.S. government to supply 10 million treatment courses…

On Nov. 17, 2021, Novavax and and Serum Institute of India announced that the Philippine Food and Drug…