Health Canada authorized Novavax COVID-19 vaccine
On Feb. 17, 2022, Novavax announced that Health Canada had granted authorization for Nuvaxovid COVID-19 Vaccine (Recombinant protein,…

On Feb. 17, 2022, Novavax announced that Health Canada had granted authorization for Nuvaxovid COVID-19 Vaccine (Recombinant protein,…

On Feb. 15, 2022, Pfizer announced that the European Medicines Agency had approved the companyメs 20-valent pneumococcal conjugate…
On Feb. 15, 2022, a woman with HIV who received a cord blood stem cell transplant to treat…

On Feb. 14, 2022, Novavax announced that the Singapore Health Sciences Authority (HSA) had issued interim authorization for…

On Feb. 14, 2022, Novavax announced its submission to Swissmedic, the Swiss Agency for Therapeutic Products, for conditional…

On Feb. 14, 2022, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) confirmed…

On Feb. 14, 2022, the U.S. Centers for Disease Control and Prevention (CDC) reported that the current risk…
On Feb. 12, 2022, China’s medical products regulator announced that it had given conditional approval for Pfizer’s COVID-19…
On Feb. 11, 2022, Gilead Sciences announced new data from an interim analysis of its ongoing, Phase 2/3…
On Feb. 11, 2022, Gilead Sciences announced new data from an interim analysis of its ongoing, Phase 2/3…

On Feb. 10, 2022, Novavax announced that NVX-CoV2373, its recombinant nanoparticle protein-based COVID-19 vaccine, achieved its primary effectiveness…

On Feb. 9, 2022, the Indiana Department of Health confirmed that a case of H5N1 highly pathogenic avian…
On Feb. 8, 2022, Moderna announced a new supply agreement with the government of Colombia for 10.8 million…

On Feb. 8, 2022, Merck and Ridgeback Biotherapeutics announced that a total of 3.1 million courses of molnupiravir,…

On Feb. 8, 2022, a research team led at Boston University School of Public Health reported on links…

On Feb. 8, 2022, researchers at Oxford University announced they had implanted a novel closed-loop research platform for…
On Feb. 7, 2022, researchers at Washington University School of Medicine in St. Louis announced an analysis of…

On Feb. 4, 2022, the Advisory Committee on Immunization Practices (ACIP) issued a standard recommendation for use of…

On Feb. 3, 2022, T2 Biosystems announced that its T2SARS-CoV-2 Panel detected Omicron COVID-19 subvariants BA.1, BA.2, and…

On Feb. 3, 2022, Novavax announced that New Zealand’s Medsafe had been granted provisional approval of NVX-CoV2373, Novavax’…
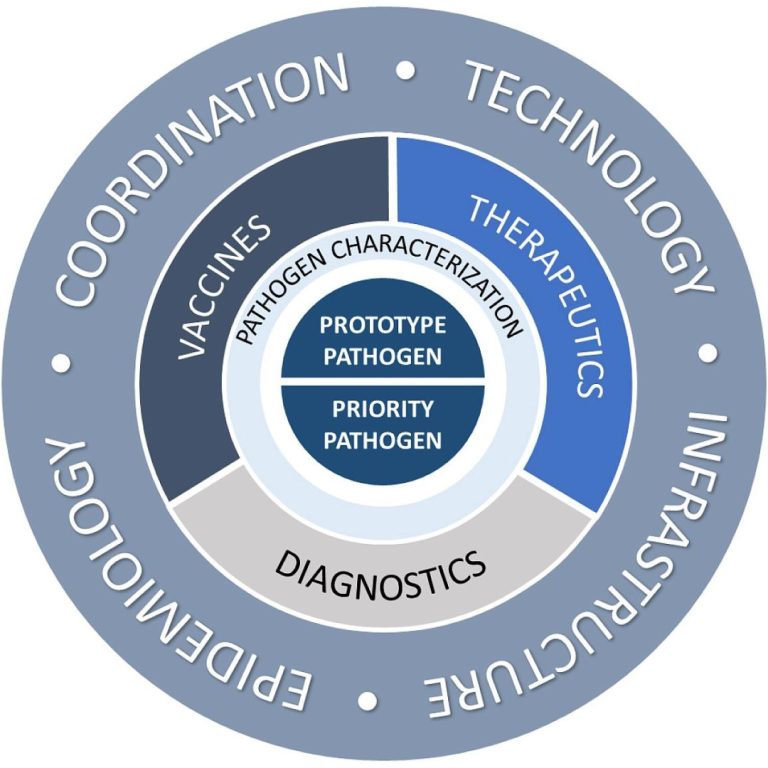
On Feb. 2, 2022, NIAID scientists announced the new Pandemic Preparedness Plan aimed to support critical basic and…

On Feb. 2, 2022, a new analysis of the first two patients treated in a clinical trial with…
On Feb. 2, 2022, President Biden announced a reignition of the Cancer Moonshot, that highlighted new goals: to…

On Jan. 31, 2022, Moderna announced the U.S. Food and Drug Administration (FDA) had approved the Biologics License…

On Jan. 31, 2022, Novavax announced that it had submitted a request to the U.S Food and Drug…

On Jan. 28, 2022, Novavax and Israel’s Ministry of Health announced an agreement for the purchase of NVX-CoV2373,…

On Jan. 28, 2022, Merck and Ridgeback Biotherapeutics announced data from six preclinical studies demonstrating that molnupiravir, an…

On Jan. 27, 2022, Cocrystal Pharma announced that it had selected two investigational novel antiviral drug candidates for…

On Jan. 26, 2022, Moderna announced that the first participant had been dosed in the Phase 2 study…

On Jan. 26, 2022, a former University of British Columbia post-doctoral research fellow led an international research team…