COVID-causing virus in air detected with high-tech bubbles
On Oct. 25, 2022, Pacific Northwest National Laboratory (PNNL) announced that scientists have shown that they can detect…
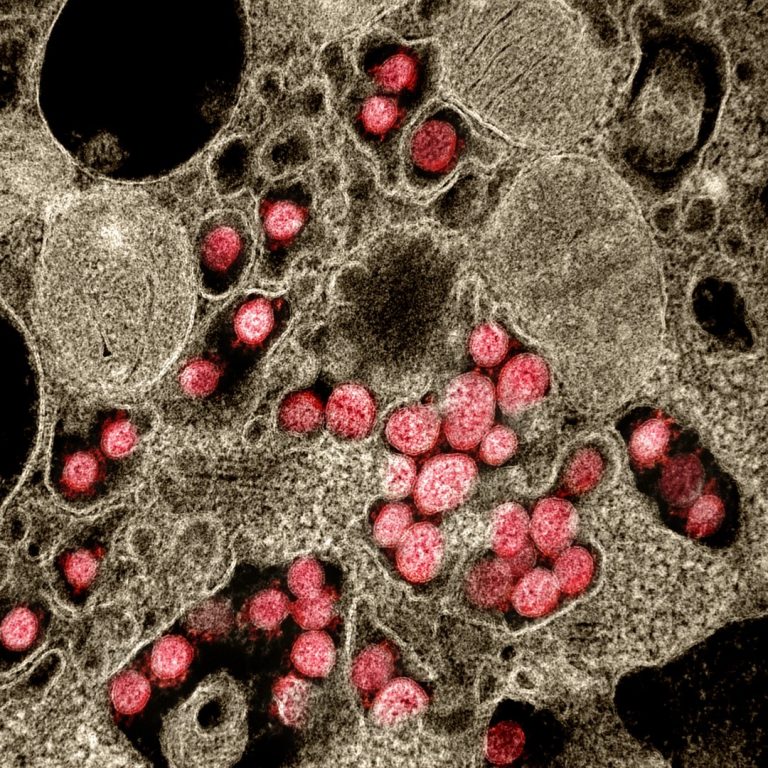
On Oct. 25, 2022, Pacific Northwest National Laboratory (PNNL) announced that scientists have shown that they can detect…
On Oct. 24, 2022, the National Institutes of Health announced that starting antiretroviral treatment (ART) early in the…
On Oct. 20, 2022, the National Institutes of Health announced that a three-dose course of the hepatitis B…
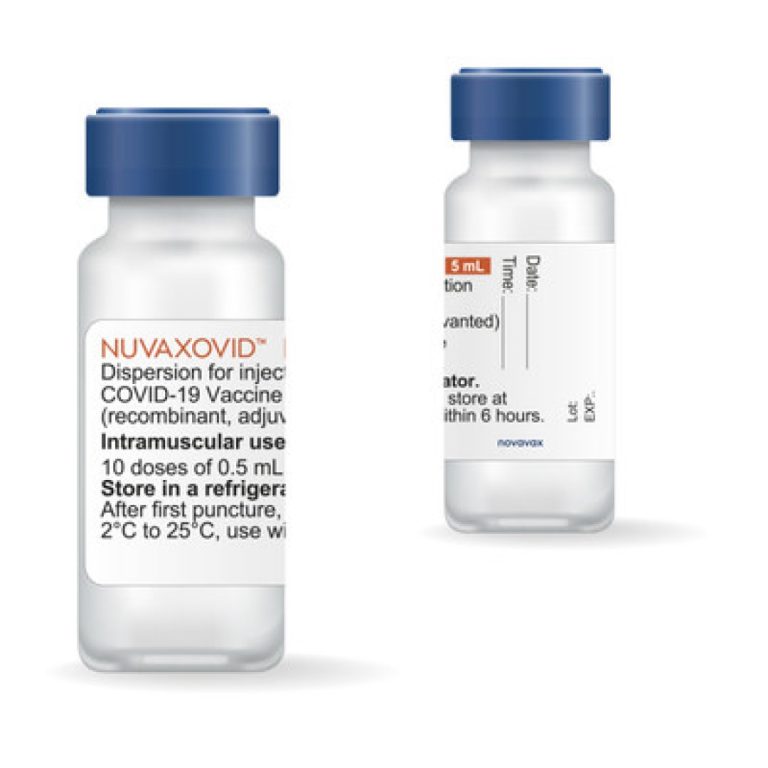
On Oct. 19, 2022, the U.S. Food and Drug Administration (FDA) recommended the use of the Novavax COVID-19…

On Oct. 19, 2022, Moderna announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human…

On Oct. 19, 2022, Moderna announced new clinical data on its bivalent Omicron-containing booster, mRNA-1273.214. Ninety days after…

On Oct. 18, 2022, global leaders confirmed US$ 2.6 billion in funding toward the Global Polio Eradication Initiative’s…

On Oct. 17, 2022, Moderna and Gavi, the Vaccine Alliance, announced they had mutually agreed to cancel remaining…
On Oct. 13, 2022, Novavax announced positive results from the Phase 1/2 clinical trial of its COVID-19-Influenza Combination…
On Oct. 13, 2022, Pfizer and BioNTech announced early data from a Phase 2/3 clinical trial (NCT05472038) evaluating…

On Oct. 13, 2022, a team of researchers led by Cornell University announced they had sequenced the Honeycrisp…

On Oct. 12, 2022, the Fred Hutchinson Cancer Center (FHCRC) announced the Bezos family had committed $710.5 million…
On Oct. 12, 2022, Moderna announced that it had received emergency use authorization from the U.S. Food and…

On Oct. 12, 2022, Pfizer and BioNTech announced that the U.S. Food and Drug Administration granted Emergency Use…

On Oct. 10, 2022, Novavax announced that partner, SK bioscience, had submitted a Post Approval Change Application to…
On Oct. 10, 2022, Novavax announced that Switzerland’s Federal Office of Public Health had recommended Nuvaxovid (NVX-CoV2373) as…
On Oct. 10, 2022, the Mayo Clinic announced that The Fred C. and Katherine B. Andersen Foundation of…
On Oct. 7, 2022, Pfizer and BioNTech announced that Health Canada had authorized COMIRNATY Original & Omicron BA.4/BA.5…

On Oct. 7, 2022, the U.S. Department of Agricultures Animal Plant Health Inspection Service (APHIS) confirmed the presence…

On Oct. 6, 2022, researchers in Japan released a study that described the development and application of the…

On Oct. 4, 2022, the United States Department of Agriculture’s (USDA) Agricultural Research Service (ARS), Southeast Poultry Research…

On Oct. 3, 2022, the Nobel Prize in Physiology or Medicine was awarded to was awarded to Svante…
On Sept. 29, 2022, the World Health Organization announced that it had identified the spread of Anopheles stephensi…

On Sept. 28, 2022, Moderna announced that the European Medicines Agency (EMA) had accepted a variation for the…

On Sept. 28, 2022, the Oregon Department of Agriculture and the U.S. Department of Agriculture’s Animal Plant Health…
On Sept. 27, 2022, the National Institutes of Health (NIH) announced that a large international study had confirmed…

On Sept. 27, 2022, Novavax announced that an initial one million doses of Nuvaxovid (NVX-CoV2373) COVID-19 vaccine were…
On Sept. 27, 2022, the National Institutes of Health (NIH) announced it had launched a program to better…
On Sept. 28, 2022, Pfizer and BioNTech announced they have completed a submission to the U.S. Food and…

On Supt. 22, 2022, Pfizer announced an agreement to supply up to six million treatment courses of its…