Vertex and CRISPR Therapeutics announced U.S. FDA approval of CASGEVY for treatment of sickle Cell disease
On Dec. 8, 2023, Vertex Pharmaceuticals and CRISPR Therapeutics announced that the U.S. Food and Drug Administration (FDA)…

On Dec. 8, 2023, Vertex Pharmaceuticals and CRISPR Therapeutics announced that the U.S. Food and Drug Administration (FDA)…

On Dec. 8, 2023, bluebird bio announced the U.S. commercial launch of its LYFGENIA’ (lovotibeglogene autotemcel, also known…
On Dec. 6, 2023, Anixa Biosciences announced updated positive results from the Phase 1 clinical trial of its…

On Dec. 5, 2023, Novavax announced that Health Canada had granted expanded authorization for Nuvaxovid XBB.1.5 Vaccine (Recombinant…

On Dec. 4 2023, the University of Wisconsin-Madison researchers announced that while analyzing data from wastewater samples collected…
On Dec. 1, 2023, the World Health Organization (WHO) announced that the International Health Regulations National Focal Point…

On Nov. 30, 2023, in a momentous landmark for medical research, UK Biobank announced that it had unveiled…
On Nov. 29, 2023, Novavax’s partner, SK bioscience, announced that Novavax’s updated COVID-19 vaccine (NVX-CoV2601) had received Emergency…

On Nov. 29, 2023, the World Health Organization (WHO) announced that between 24 and 25 November 2023, the…

On Nov. 28, 2023, Novavax announced that Nuvaxovid XBB.1.5 COVID-19 Vaccine (NVX-CoV2601) had been granted Emergency Use Listing…
On Nov. 28, 2023, Emergent BioSolutions announced that the Biomedical Advanced Research and Development Authority (BARDA) within the…
On Nov. 27, 2023, University of Wisconsin-Madison researchers released a study that showed COVID-19 caused an alarming surge…

On Nov. 23, 2023, the World Health Organization (WHO) announced that it was monitoring data from Chinese surveillance…

On Nov. 17, 2023, the Oregon Department of Agriculture reported that the United States Department of Agriculture’s National…

On Nov. 17, 2023, Masimo announced that the Masimo W1 medical watch has received FDA 510(k) clearance for…

On Nov. 16, 2023, Vertex and CRISPR Therapeutics announced that the United Kingdom (U.K.) Medicines and Healthcare products…

On Nov. 15, 2023, the Cancer Prevention and Research Institute of Texas (CPRIT) announced that it had approved…

On Nov. 14, 2023, Yellowstone National Park and the Wyoming Game and Fish Department (WGFD) officials confirmed the…

On Nov. 13, 2023, the World Health Organization (WHO announced that the Ministry of Health and Social Welfare…

On Nov. 13, 2023, the White House announced the first-ever Initiative on Womenメs Health Research, an effort led…
On Nov. 10, 2023, the Centers for Disease Control and Prevention (CDC) estimated that from October 1, 2023,…
On Nov. 6, 2023, researchers at NYU Grossman School of Medicine announced they had engineered the first mice…

On Oct. 31, 2023, Novavax announced that the European Commission had granted approval for Nuvaxovid XBB.1.5 dispersion for…
On Oct. 30, 2023,California Department of Public Health reported The first locally acquired case of dengue in California…
On Oct. 27, 2023, the California Institute for Regenerative Medicine (CIRM) awarded $42.1 million to fund various translational…

On Oct. 25, 2023, University of British Columbia researchers announced they had identified buried kimberlite, the rocky home…

On Oct. 23, 2023, the British Antarctic Survey (BAS) reported that Highly Pathogenic Avian Influenza (HPAI) had been…
On Oct. 20, 2023, the U.S. Centers for Disease Control and Prevention (CDC) reported that among children and…
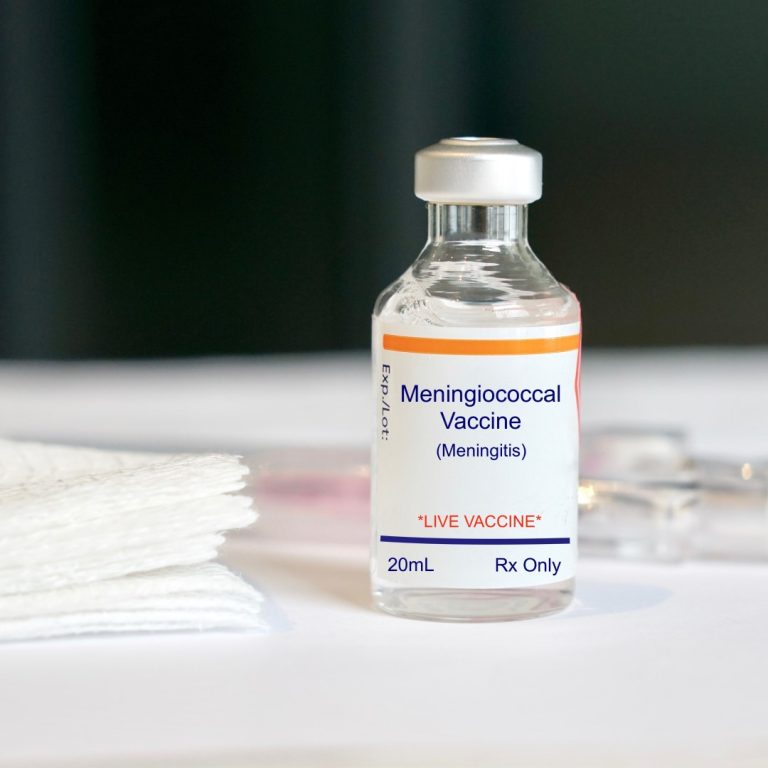
On Oct. 20, 2023, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved PENBRAYA (meningococcal…
On Oct. 18, 2023, Novavax announced that the Medicines and Healthcare products Regulatory Agency (MHRA) in the United…