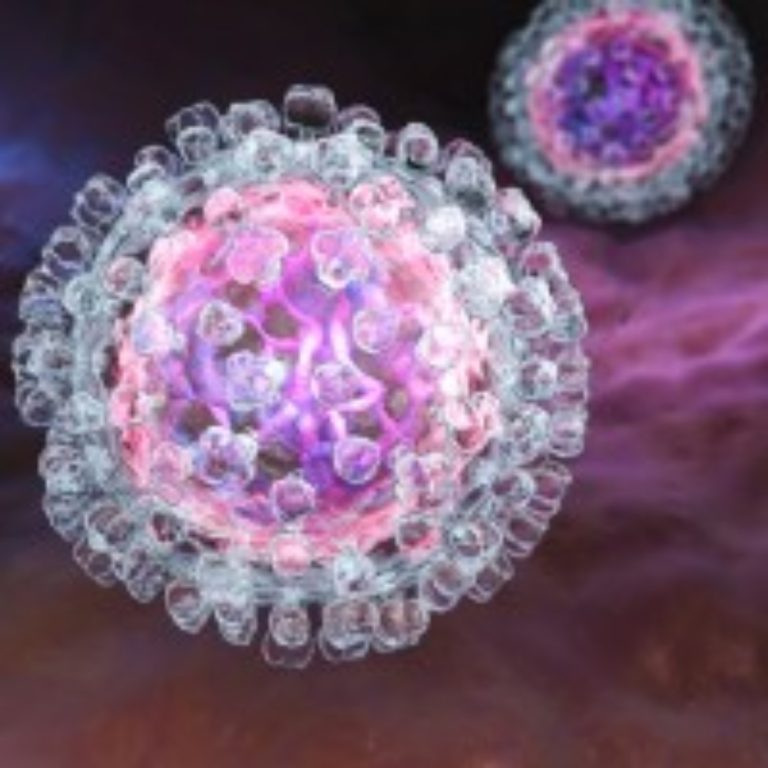
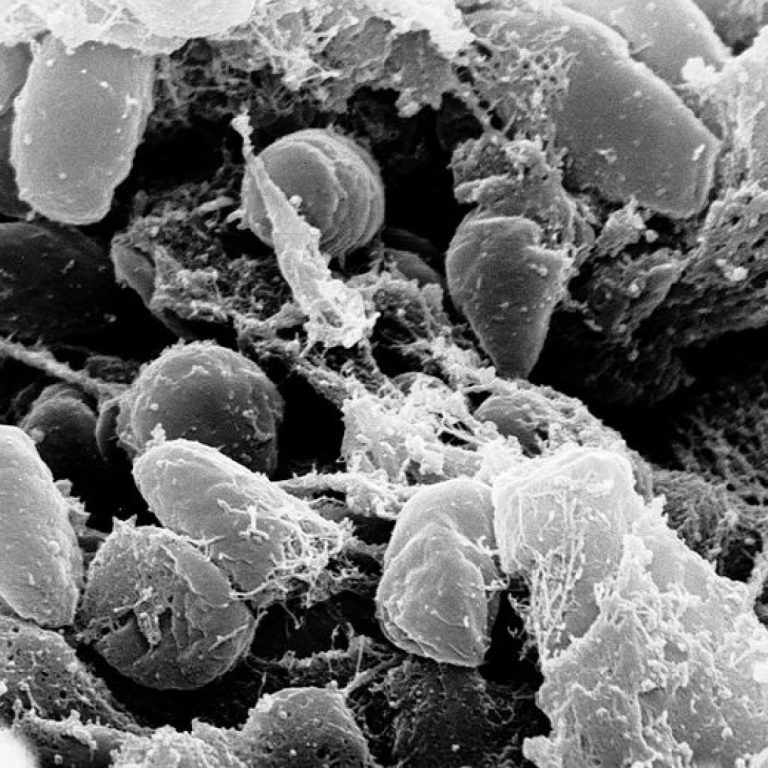
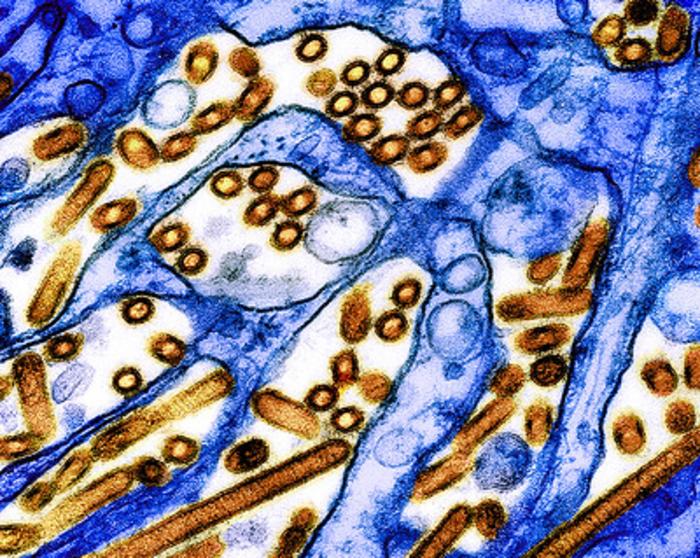
Maryland Department of Health issued consumer advisory for Boar’s Head liverwurst and other deli meat products due to possible Listeria contamination
On Jul. 26, 2024, the Maryland Department of Health advised consumers not to eat certain deli meats produced…