Multi-year, 1143-patient genetic study identified unique types of multiple myeloma
On Aug. 19, 2024, an international team of scientists led by researchers from the Translational Genomics Research Institute (TGen) announced…
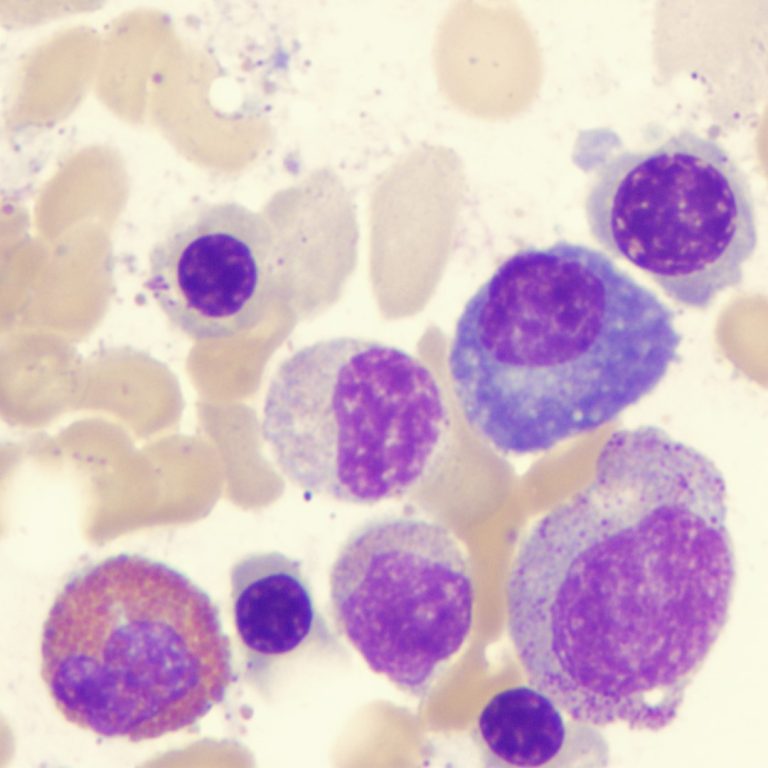
On Aug. 19, 2024, an international team of scientists led by researchers from the Translational Genomics Research Institute (TGen) announced…

On Aug. 16, 2024, the U.S. Centers for Disease Control and Prevention (CDC) reported that COVID-19 infections are…
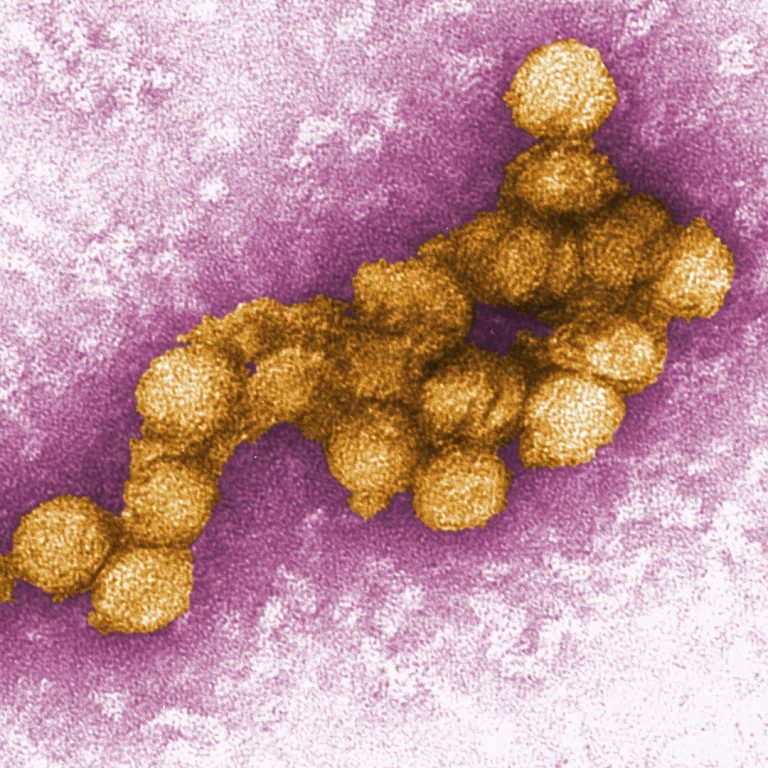
ON Aug. 16, 2024, the European Centre for Disease Prevention and Control (ECDC) reported that it is highly…

On Aug. 15, 2024, researcher from from Western University announced they had discovered a protein that has the…
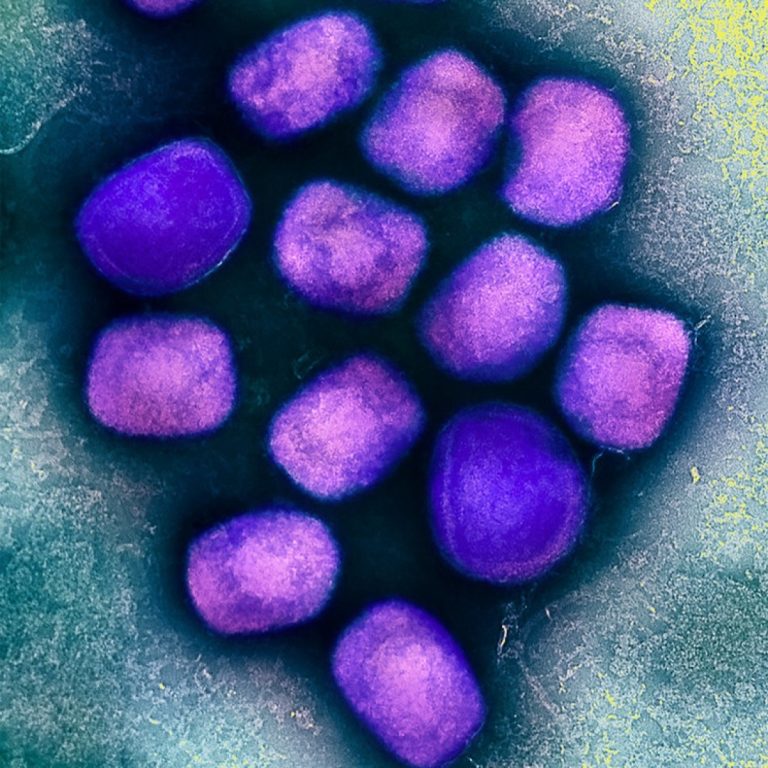
On Aug. 15, 2024, the Oakland County Health Division in Pontiac, Michigan urged residents to protect themselves from mosquito…

On Aug. 15, 2024, a study published in the journal Cancer projected an alarming increase in cancer cases and deaths…
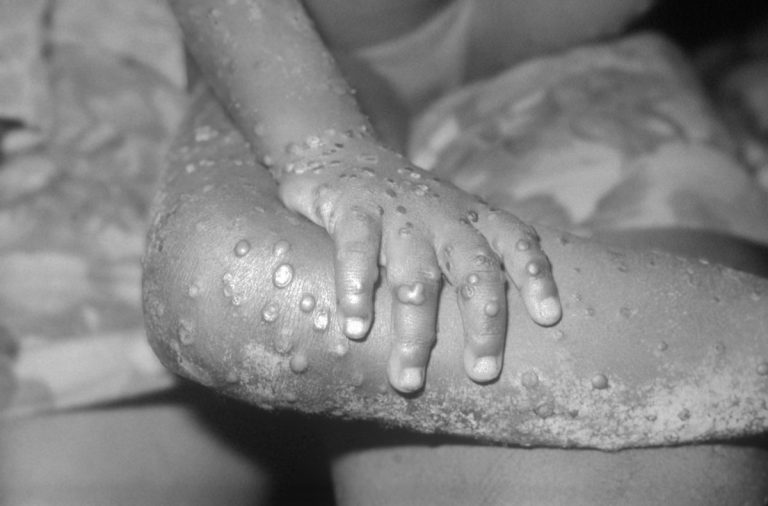
On Aug. 14, 2024, The World Health Organization declared that the upsurge of mpox in the Democratic Republic…

On Aug. 14, 2024, scientists from Uruguay University announced that a species of lungfish found in South America…
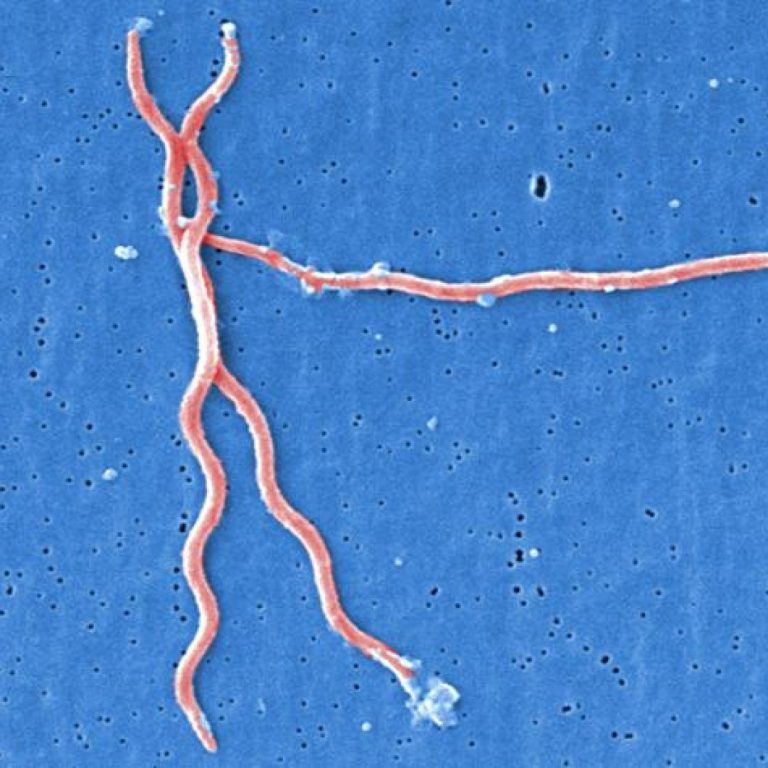
On Aug. 14, 2024, an international team of scientists announced they had sequenced the complete genomes of the…
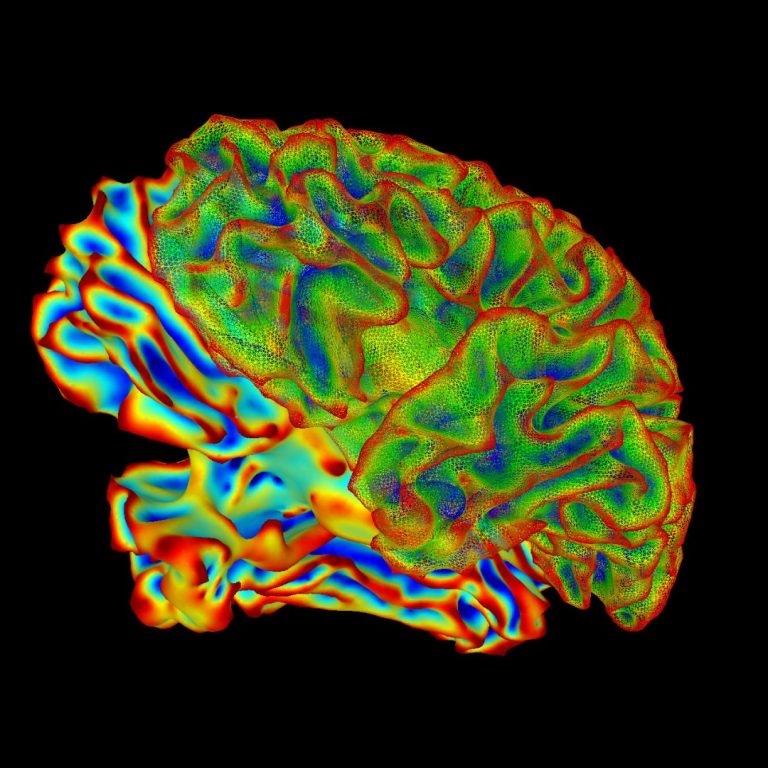
On Aug. 14, 2024, a study co-led by Mass General Brigham found that one in four patients with…

on Aug. 14, 2024, research led by scientists at the Ineos Oxford Institute for antimicrobial research at Oxford…

On Aug. 13, 2024, Eli Lilly announced the opening of the Lilly Seaport Innovation Center (LSC), a research…

On Aug. 13, 2024, President Joe Biden announced $150 million in awards from the Advanced Research Projects Agency…

On Aug. 12, 2024, The Howard Hughes Medical Institute (HHMI) announced AI@HHMI, a $500 million investment over the…
On Aug. 8, the World Health Organization (WHO) announced results of a study that showed the introduction of…
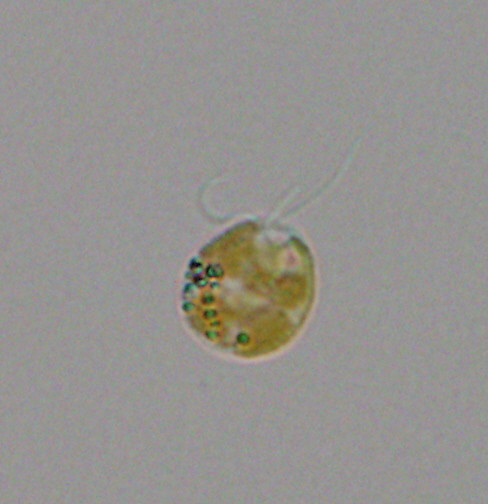
On Aug. 8, 2024, scientists at UC San Diego’s Scripps Institution of Oceanography announced they had discovered the…

On Aug. 7, 2024, Amneal Pharmaceuticals announced that the U.S. Food and Drug Administration (FDA) had approved CREXONT…
On Aug. 6, 2024, the U.S. Food and Drug Administration (FDA) announced it had approved vorasidenib (Voranigo, Servier…

On Aug. 6, 2024, a team of researchers, led by the University of Houston, announced they had discovered…

On Aug. 6, 2024, the University of Pennsylvania announced it had sued German biotechnology firm BioNTech n Pennsylvania…

On Aug. 5, 2024, North Carolina State University researchers revealed that an examination of the genetic material found…
On Aug. 5, 2024, Novartis and Viatris were named a federal lawsuit in Maryland by the family of…
On Aug. 2, 2024, the U.S. Food and Drug Administration approved Adaptimmune’s Tecelra (afamitresgene autoleucel), a gene therapy…
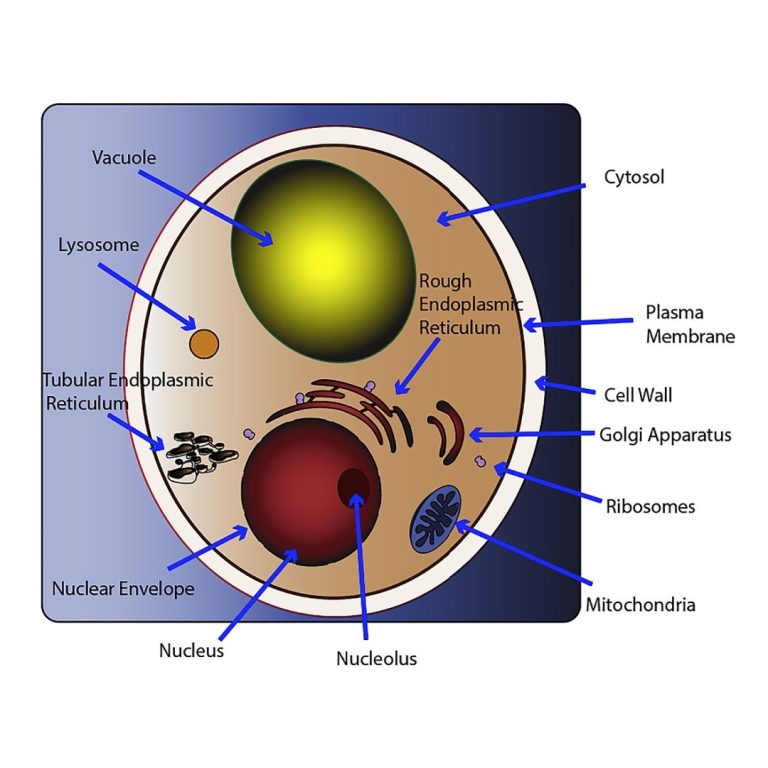
On Aug. 1, 2024, the Van Andel Institute reported that humans and baker’s yeast have more in common…

On Aug. 1, 2024, the Coalition for Epidemic Preparedness Innovations (CEPI) and the World Health Organization (WHO) called…

On Jul. 31, 2024, the Lancet Standing Commission released its 2024 Dementia prevention, intervention, and care report that…

On Jul. 31, 2024, researchers at Washington State University (WSU) announced that the cannabigerol (CBG) effectively reduced anxiety…

On Jul. 31, 2024, a large study led by researchers at the American Cancer Society (ACS) suggested incidence…
On Jul. 29, 2024, a team led by the Barbara Ann Karmanos Cancer Institute and the Wayne State…

On Jul. 29, 2024, a new project that aims to accelerate the development and accessibility of human avian…