RSV vaccine in older people reduced risk of hospitalization
On Sep. 4, 2024, researcher led by the Centers for Disease Control and Prevention (CDC) and Vanderbilt University…
On Sep. 4, 2024, researcher led by the Centers for Disease Control and Prevention (CDC) and Vanderbilt University…
On Sep. 4, 2024, the World Health Organization (WHO) published global cholera statistics for 2023, that showed an…
On Sept. 3, 2024, a major study led by the UC Davis Comprehensive Cancer Center (UC DAVIS) has…
On Sept. 3, 2024, researchers at NORC at the University of Chicago and George Washington University’s Milken Institute…

On Sep. 3, 2024, the National Cancer Institute (NCI) announced that the latest Cancer Trends Progress Report included…

On Sep. 2, 2024, the World Health Organization (WHO) confirmed that a laboratory-confirmed case of human infection with…

On Aug. 30, 2024, Novavax announced the Novavax COVID-19 Vaccine, Adjuvanted (2024-2025 Formula) (NVX-CoV2705) had received Emergency Use…
On Aug. 30, 2024, the California Institute for Regenerative Medicine (CIRM) approved $67.5 million to support five projects…
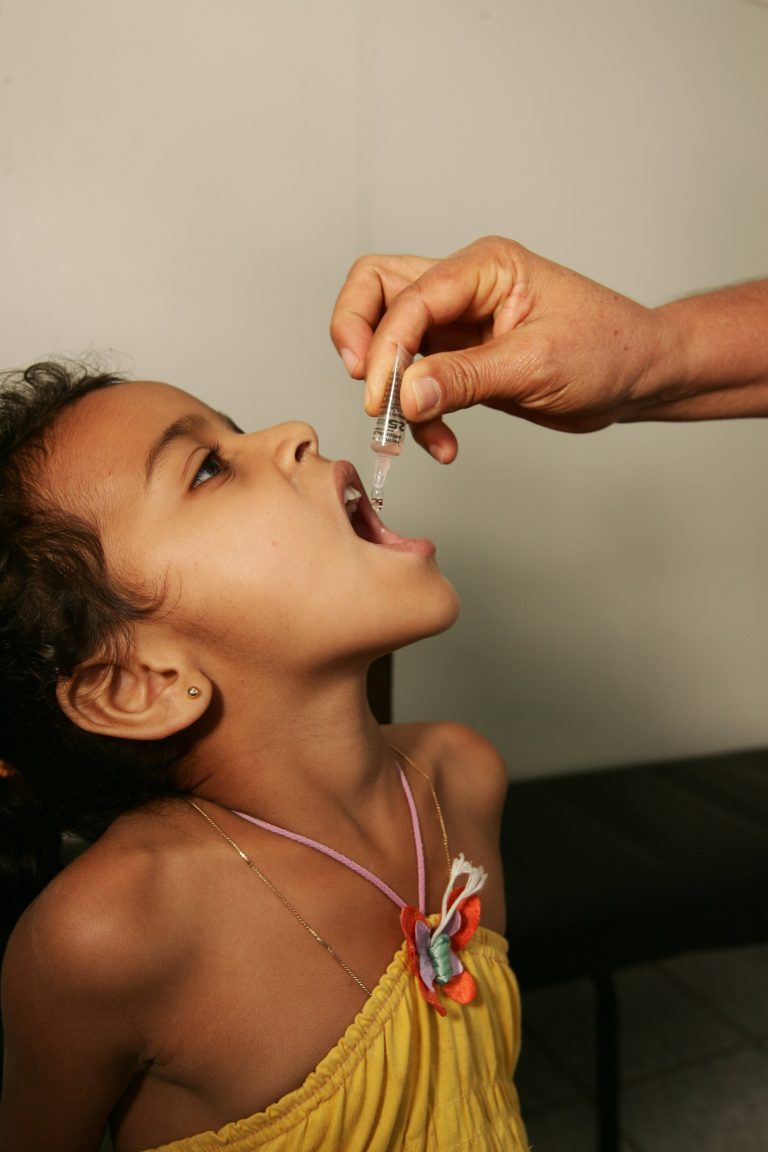
On Aug. 30, 2024, the United Nations (UN) announced that polio vaccination will begin for some 640,000 children…
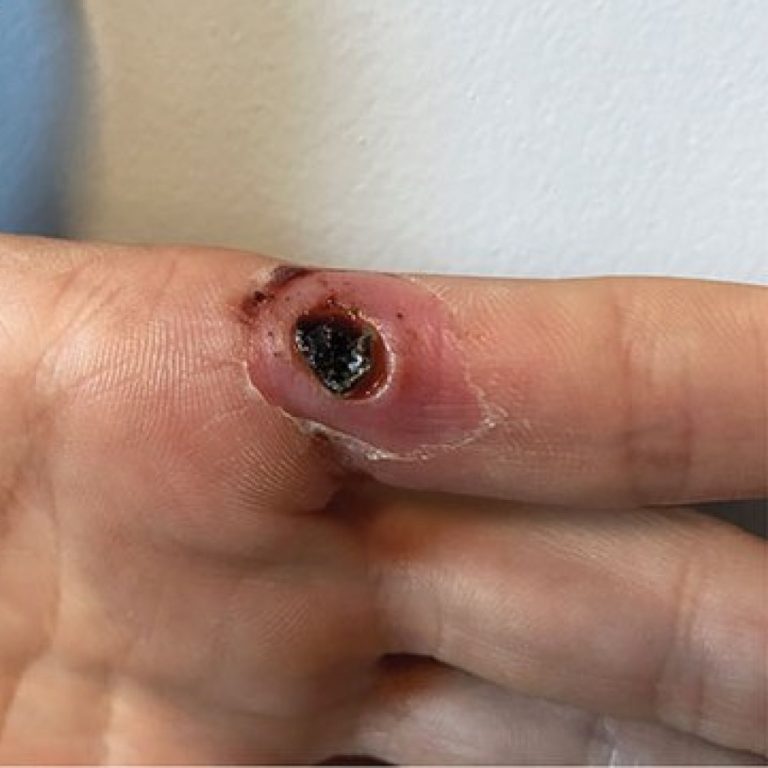
On Aug. 29, 2024, Emergent BioSolutions announced that the U.S. Food and Drug Administration (FDA) had approved the supplemental Biologics…
On Aug. 29, 2024, researchers at the Garvan Institute of Medical Research announced they had mapped 50,000 of…
On Aug. 29, 2024, a small team of scientists has launched an open-source database for some of the…

On Aug. 28, 2024, the University of Würzburg announced research using newly generated “optogenetic” tobacco plants to investigated…
On Aug. 28, 2024, survey data from the Annenberg Public Policy Center (APPC) found that the number of…

On Aug. 28, 2024, Northwestern University and University of Texas-Southwestern announced results of a study that found bacterial…

On Aug. 27, 2024, the New Hampshire Department of Health and Human Services (DHHS) identified an adult who…

On Aug. 26, 2024, the University of Michigan (UM) announced that one in five parents say their child…
On Aug. 26, 2024, the World Health Organization (WHO) launched a global Strategic Preparedness and Response Plan to…

On Aug. 23, 2024, the U.S. Centers for Disease Control and Prevention (CDC) reported that two human infections…

On Aug. 23, 2024, the World Health Organization announced that between early June and 15 August 2024, the…
On Aug. 23, 2024, A team of researchers from the University of Cologne announced they had created a…
On Aug. 22, 2024, Pfizer and BioNTech announced that the U.S. Food and Drug Administration (FDA) had approved…

On Aug. 23, 2024, the World Health Organization (WHO) reported an outbreak of Oropouche virus (OROV) in the…

On Aug. 21, 2024, researchers at the University of Minnesota (UM) announced a first-of-its-kind adaptive 3D printing system…
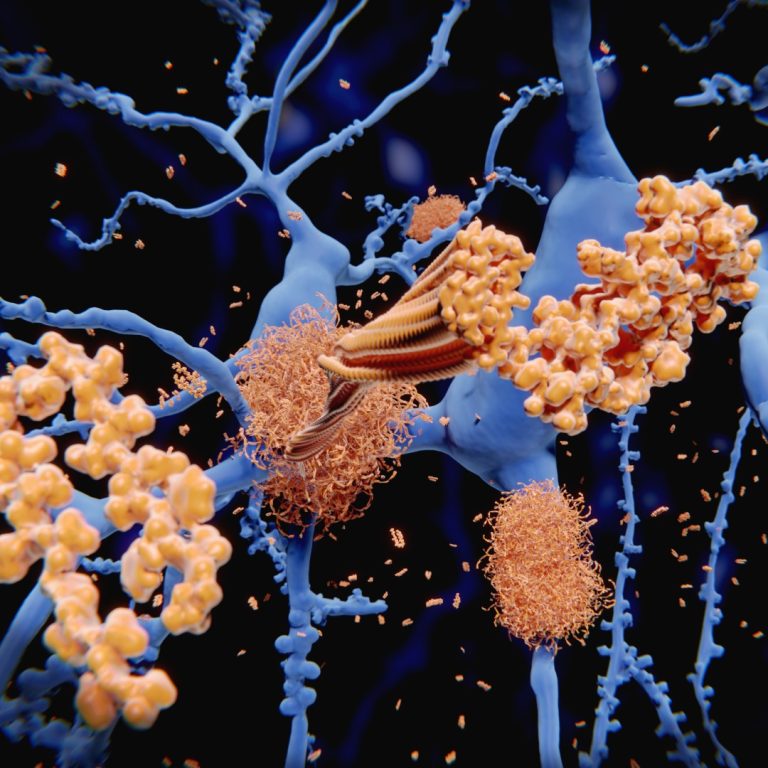
On Aug. 22, 2024, the Medicines and Healthcare Products Regulatory Agency (MHRA) announced it had licensed the immunotherapy…
On Aug. 21, 2024, Contrary to previous research, a new study of female participants finds no link between…
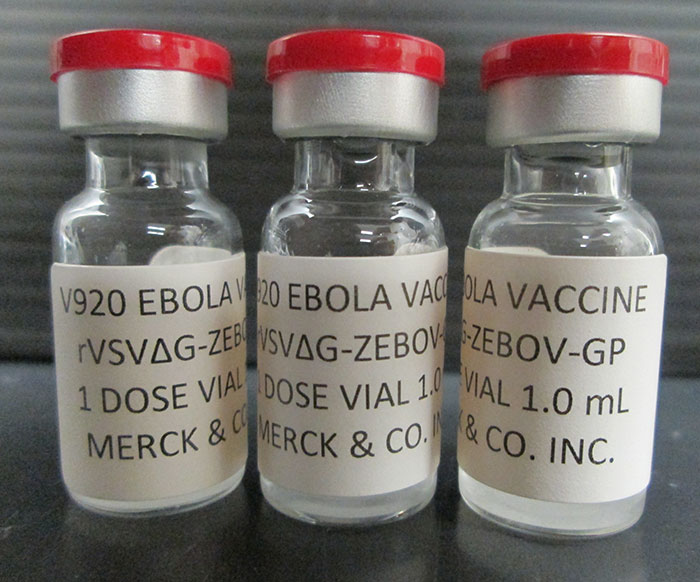
On Aug. 21, 2024, French and Democratic Republic of the Congo (DRC) researchers announced that a real-world study…

On Aug. 21, 2024, the Cancer Prevention and Research Institute of Texas (CPRIT) announced that it had approved…
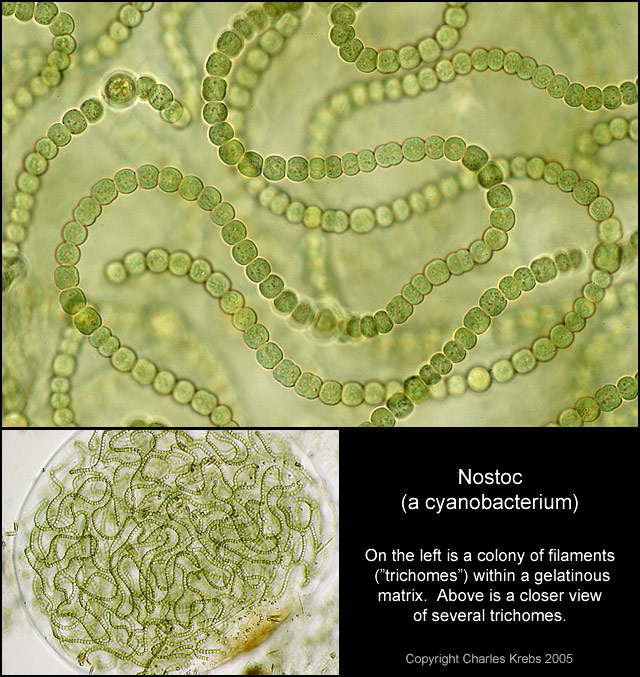
On Aug. 21, 2024, a research team at the University of Texas at Austin reported that the first…
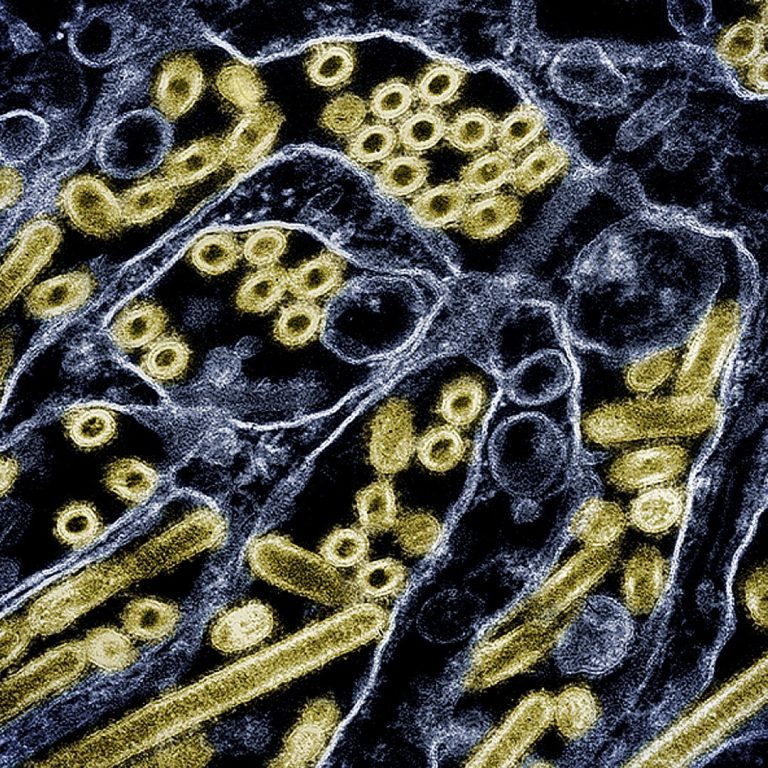
On Aug. 19, 2024, scientists at the European Molecular Biology Laboratory (EMBL) published research that sheds light on…