WHO verified Jordan as first country to eliminate leprosy
On Sept. 19, 2024, the World Health Organization (WHO) announced that Jordan had become the first country in…
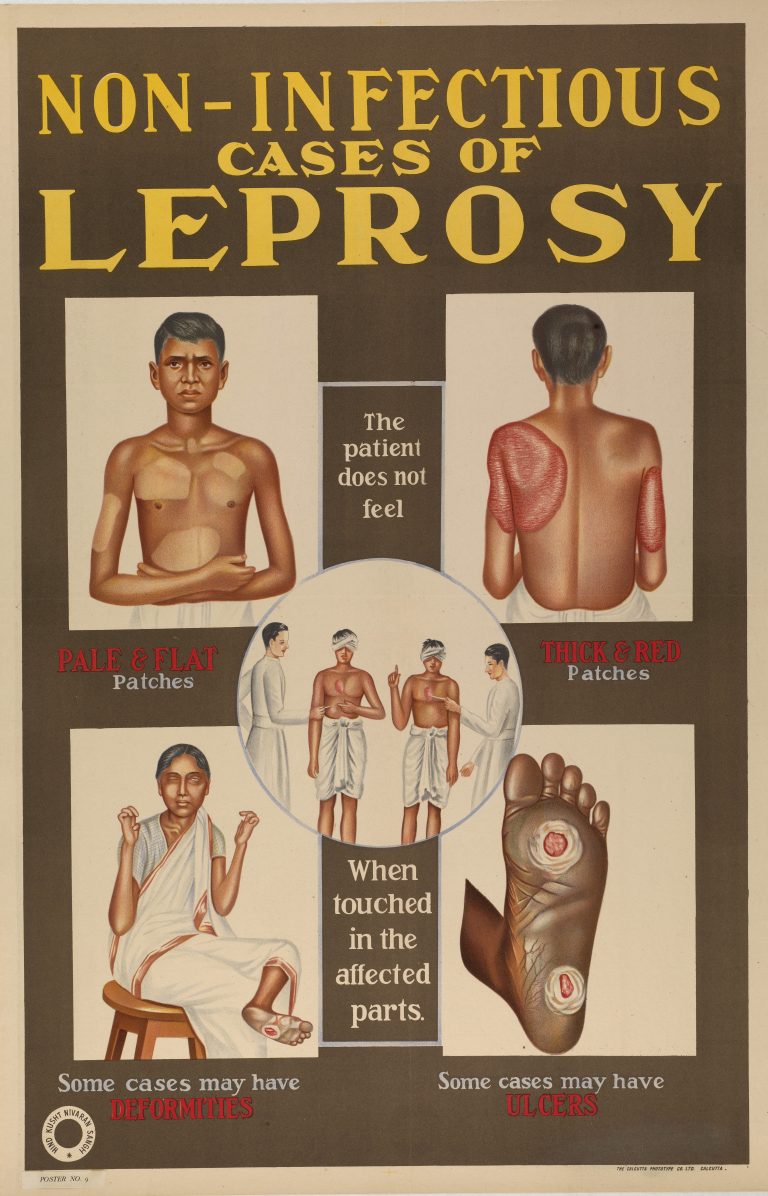
On Sept. 19, 2024, the World Health Organization (WHO) announced that Jordan had become the first country in…
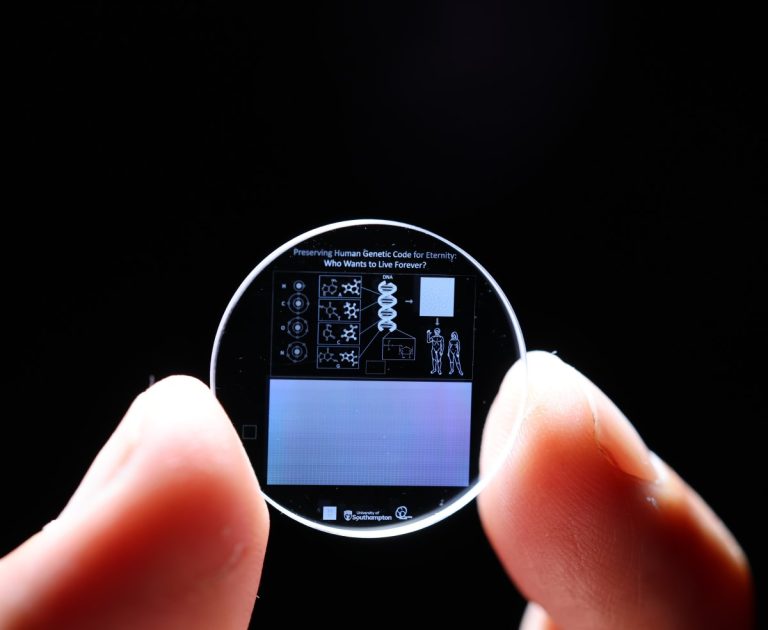
On Sept. 19, 2024, scientists from the University of Southampton’s Optoelectronics Research Centre announced they had stored the…

On Sept. 19, 2024, scientists from Sun Yat-Sen University in China announced they had revived a pig’s brain…

On Sept. 18, 2024, a study published in The Lancel reported that here has been a rapid rise…
On Sept. 18, 2024, researchers from the Wellcome Sanger Institute and their collaborators reported that they had identified…

On Sept. 17, 2024, in landmark research, scientists at The University of Texas Health Science Center at San…

On Sept. 17, 2024, a team of researchers from the Food Packaging Forum announced that a peer-reviewed scientific…

On Sept. 17, 2024, researchers from Tufts University announced they had received a $20.7 million grant from the…
On Sept. 16, 2024, researchers from NHS Blood and Transplant (Bristol), NHSBT’s International Blood Group Reference Laboratory (IBGRL)…

On Sept. 16, 2024, a study by the Global Research on Antimicrobial Resistance (GRAM) Project reported that antimicrobial…
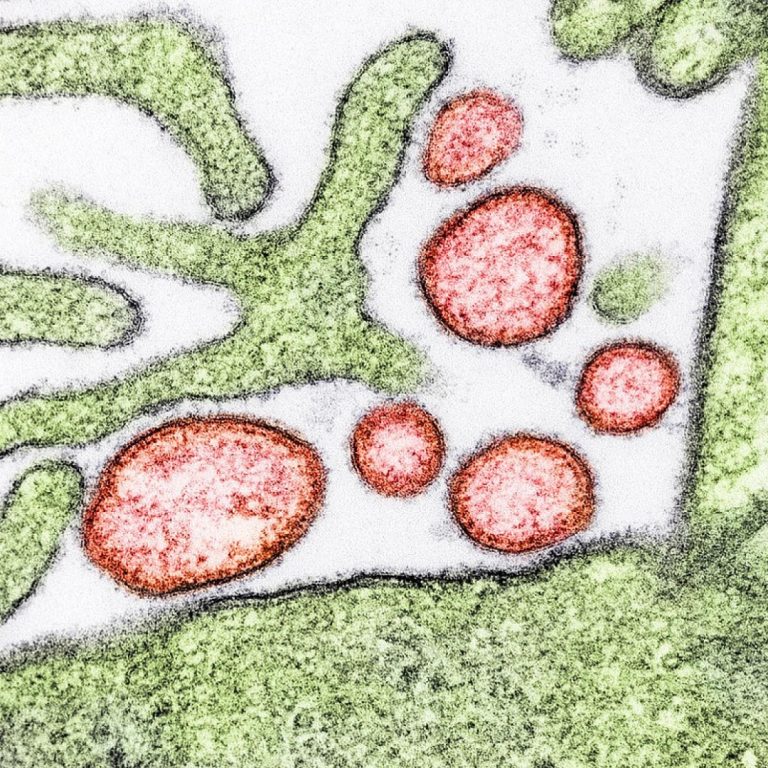
On Sept. 16, 2024, A 24-year-old student has died from the Nipah virus in the southern Indian state…

On Sep. 13, 2024, a Cleveland Clinic study was announced that identified key factors that can impact the…

On Sept. 13, 2024, researchers from Weill Cornell Medicine have used a cutting-edge model system to uncover the…

On Sep. 13. 2024, Novavax announced that doses of the Novavax COVID-19 Vaccine, Adjuvanted (2024-2025 Formula) (NVX-CoV2705) to…

On Sept. 13, 2024, researchers from the Wellcome Sanger Institute announced they had sequenced the genome of the…

On Sept. 13, 2024, Genentech announced that the U.S. Food and Drug Administration (FDA) had approved Ocrevus Zunovo™…

On Sept. 13, 2024, CSL Seqirus and sa-mRNA pioneer Arcturus Therapeutics announced that Japan’s Ministry of Health, Labor…

On Sept. 12, 2024, the Finnish Institute for Health and Welfare (THL) reported that Poliovirus had been found…

On Sep. 12, 2024, the the U.S. Food and Drug Administration (FDA) authorized the first over-the-counter (OTC) hearing…

On Sep. 11, 2024, the Translational Genomics Research Institute (TGen), part of City of Hope, announced the launch…

On Sept. 11, 2024, an international team of scientists led by the Leibniz Institute on Aging announced they…

On Sep. 11, 2024, researchers at UTHealth Houston and Baylor College of Medicine reported that Avian influenza A(H5N1)…
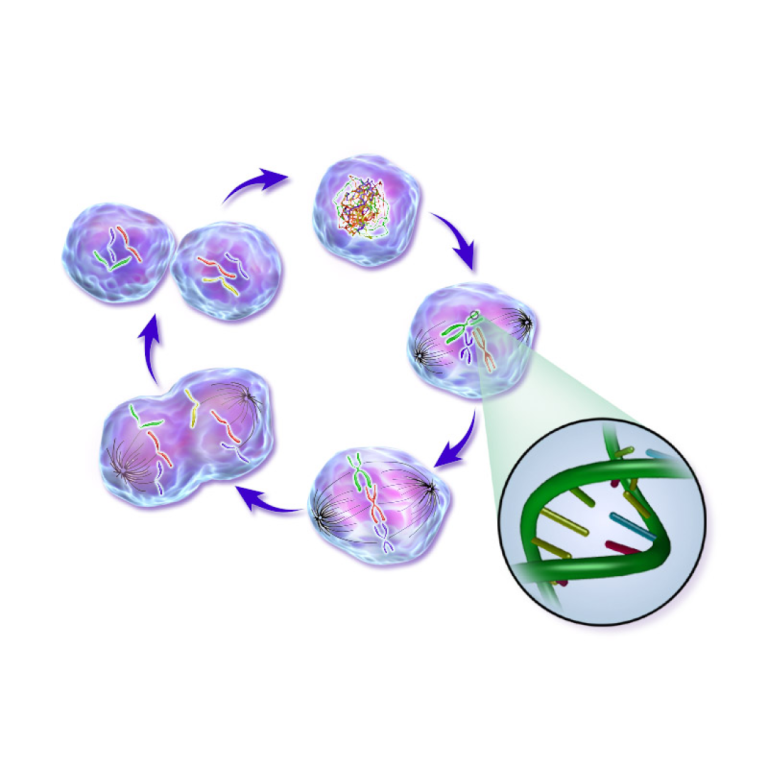
On Sep. 10, 2024, researchers at Weill Cornell Medicine in Qatar announced they had created a free-to-access online…

On Sep. 10, 2024, researchers at the Karolinska Institute in Sweden announced a study that shows that gene…
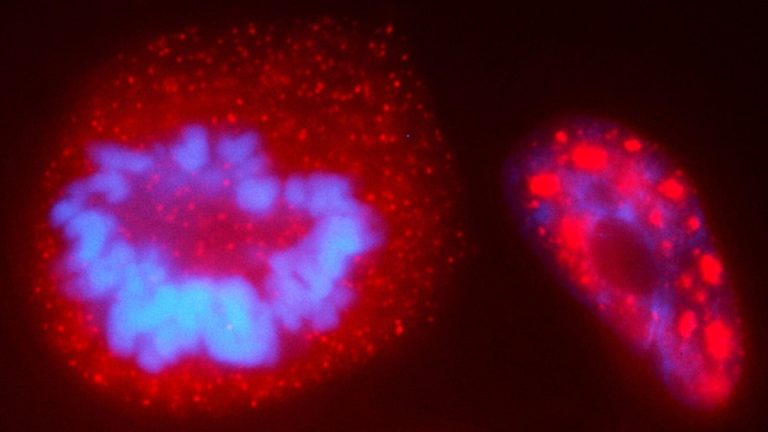
On Sep. 9, 2024, a Princeton University team announced they had developed a way to probe chromosomes and…

On Sep. 9, 2024, Santo Fortunato, a professor at Luddy School of Informatics, reported that an international research…
On Sep. 6, 2024, researchers from the Wellcome Sanger Institute, University College London (UCL), and the University of…

On Sep. 6, 2024, the Centers for Disease Control and Prevention (CDC) confirmed a human case of avian…

On Sep. 4, 2024, researchers at the University of Copenhagen announced the discovery of a gene that has…

On Sep. 4, 2024, an international consortium led by scientists from Uppsala University in Sweden reported that the…