Study Found Persistent Infection Could Explain Long COVID in Some People
On Oct. 9, 2024, researchers from Brigham and Women’s Hospital found that people with wide-ranging long COVID symptoms…
On Oct. 9, 2024, researchers from Brigham and Women’s Hospital found that people with wide-ranging long COVID symptoms…
On Oct. 9, 2024, scientists from Oregon Health & Science University (OHSU) released a study that used imaging…

On Oct. 9, 2024, in a Northwestern University-led study, microbiologists found that showerheads and toothbrushes were teeming with…

On Oct. 9, 2024, the Nobel Foundation awarded the Nobel Prize in Chemistry: one half to David Baker,…
On Oct. 9, 2024, Novavax announced that the European Commission had granted Marketing Authorization for Novavax’s updated 2024-2025…

Oct. 9, 2024, the Seattle Fire Department (SFD) expanded its Buprenorphine Pilot Program and Seattle became the first…

On Oct. 8, 2024, the Pan American Health Organization (PAHO) announced an epidemiological alert and urged countries in…

On Oct. 8, 2024, a team of University of Essex researchers found that the secret to losing weight…

On Oct. 8, 2024, researchers from the Max Planck Institute for Plant Breeding announced they have developed a…

On Oct. 8, 2024, scientists from the Commonwealth Scientific and Industrial Research Organisation (CSIRO), Australia’s national science agency,…

On Oct. 8, 2024, Okanagan Specialty Fruits® (OSF), the developer and grower behind the innovative Arctic® apple varieties, announced that its…
On Oct. 7, 2024, Columbia University researchers announced they had developed a new optical pooled screening approach called…

On Oct. 7, 2024, The Nobel Foundation announced that Victor Ambros from Harvard University and Gary Ruvkun from…
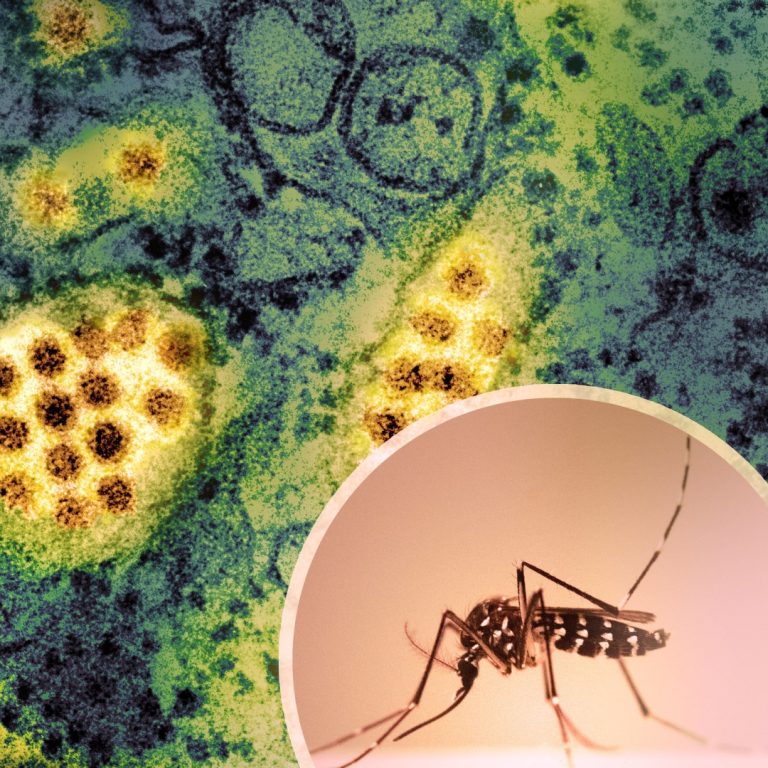
On Oct. 6, 2024, San Diego County officials announced they were investigating the first ever case of locally…
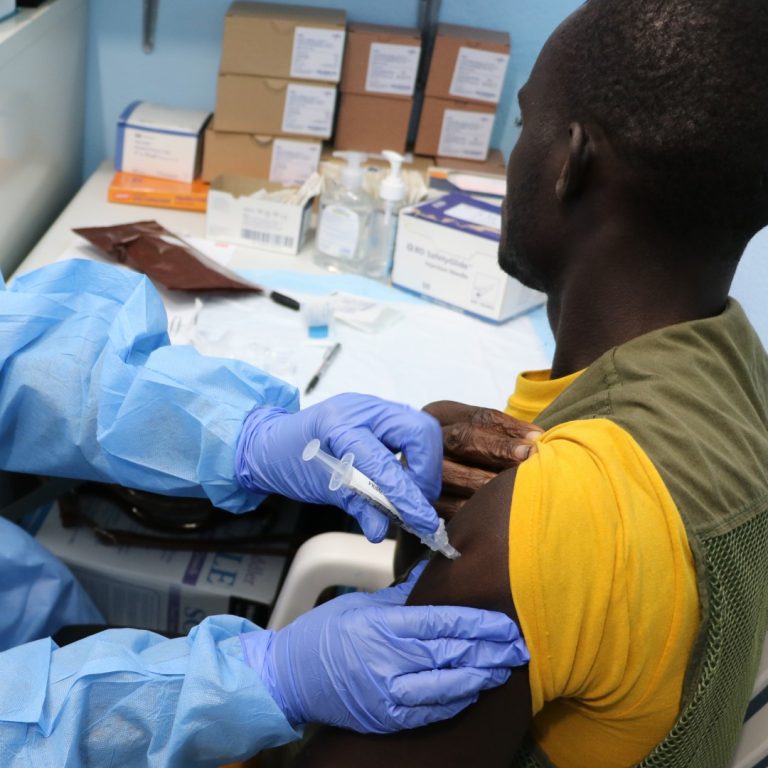
On Oct. 5, 2024, the Sabin Vaccine Institute announced it had provided its investigational Marburg vaccine to Rwanda…

On Oct. 4, 2024, researchers from Oxford university announced they had been awarded funding from Cancer Research UK…
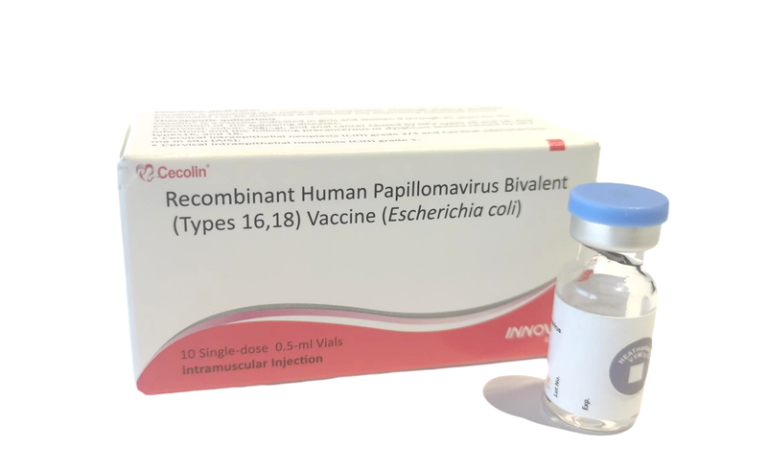
On Oct. 4, 2024, the World Health Organization (WHO) announced that a fourth WHO-prequalified human papillomavirus (HPV) vaccine…

On Oct. 4, 2024, Exact Sciences announced the U.S. Food and Drug Administration (FDA) had approved the Cologuard…
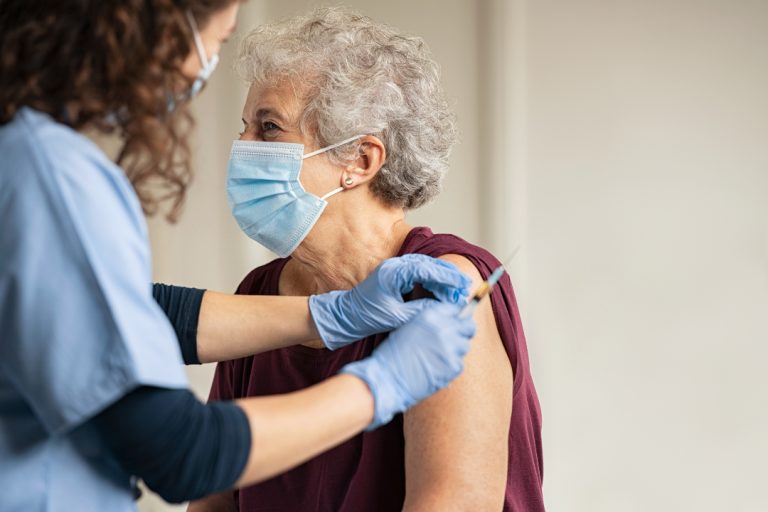
On Oct. 4, 2024, the Centers for Disease Control and Prevention (CDC) published data that showed older adults…

On Oct. 4, 2024, scientists from the the University of Chile announced they had developed an injection that…
On Oct. 3, 2024, scientists announced the largest whole-exome sequencing study of epilepsy to date, with more than…

On Oct. 3, 2024, the California Department of Public Health (CDPH) reported that the human case previously under…

On Oct. 3, 2024,the World Health Organization (WHO) launched the Global Strategic Preparedness, Readiness and Response Plan (SPRP)…
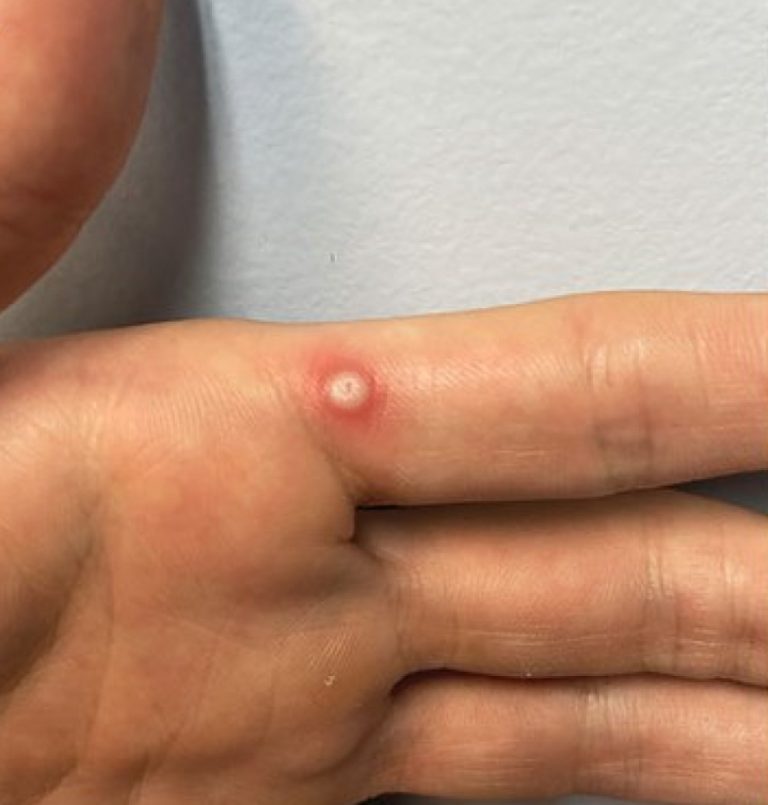
On Oct. 3, 2024, the World Health Organization (WHO) announced it had listed the first mpox in vitro…

On Oct. 2, 2024, a team of researchers from the University of Tokyo announced they had discovered living…
On Oct. 2, 2024, the World Health Organization (WHO) announced it had been notified of one human case…
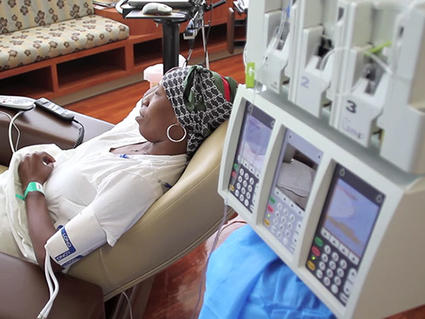
On Oct. 2, 2024, results from a French clinical trial have identified what experts say should now be…

On Oct. 2, 2024, scientists at Oregon State University (OSU) announced the release of two mild habanero peppers…

On Oct. 2, 2024, Eli Lilly and Company announced a $4.5 billion investment to create the Lilly Medicine…

On Oct. 2, 2024, a study from the GBD Tobacco Forecasting Collaborators reported that by accelerating the decline…