WHO reported Spike in outbreak of cholera
On Oct. 18, 2024, the World Health Organization (WHO) reported that from January 1 to September 29, 2024,…
On Oct. 18, 2024, the World Health Organization (WHO) reported that from January 1 to September 29, 2024,…

On Oct. 18, 2024, the California Department of Public Health (CDPH) reported that a total of 13 human…

On Oct. 18, 2024, a research team led by Radboud university medical center reported that a new PET…

On Oct. 17, 2024, Colossal Biosciences, an American biotechnology and genetic engineering company, announced that it has a…
On Oct. 17, 2024, Geisinger announced results of a study that showed increased risk for autism appears to…

On Oct. 17, 2024, a study led by the University at Buffalo and the Jackson Laboratory (JAX) revealed…

On Oct. 16, 2024, The U.S. Food and Drug Administration (FDA) announced it had updated the label for…
On Oct. 16, 2024, researchers from the Wellcome Sanger Institute, Newcastle University and their collaborators announced they had…

On Oct. 16, 2024, Know Labs in Seattle announced that it had retained The Stanbridge Group to secure…

On Oct. 16, 2024, Sanofi announced it will contribute $18 million to three Historically Black Medical Schools to…
On Oct. 16, 2024, a researchers at Copenhagen University Hospital reported in a medical case report that a…
On Oct. 16, 2024, a National Institutes of Health (NIH)-funded clinical trial of an mpox vaccine in adolescents…

On Oct. 15, 2024, Massachusetts Governor Maura Healey and the Massachusetts Life Sciences Center (MLSC) announced $21.4 million…

On Oct. 14, 2024, researchers have found a link between two human-specific genes and the gene SYNGAP1, a…

On Oct. 14, 2024, Lundbeck and Longboard Pharmaceuticals announced an agreement for Lundbeck to acquire Longboard for payment…

On Oct. 14, 2024, the Global Preparedness Monitoring Board (GPMB) released a report that outlined 15 key drivers…
On Oct, 14, 2024, a team of researchers from the Allen Institute of Brain Science and the University…

On Oct. 14, 2024, RedHill Biopharma announced that the U.S. government’s Biomedical Advanced Research and Development Authority (BARDA)…
On Oct. 12, 2024, the Sabin Vaccine Institute announced it had delivered approximately 1,000 more investigational vaccine doses…
On Oct. 11, 2024, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved HYMPAVZI™ (marstacimab-hncq)…

On Oct. 11, 2024, a team of researchers from University of Illinois Urbana-Champaign announced DNA analysis of the…
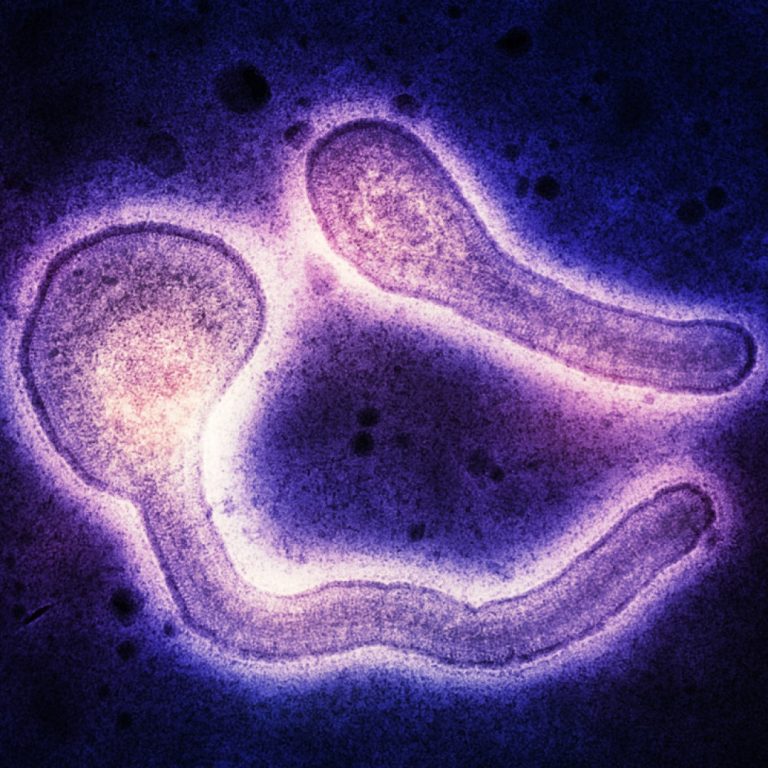
On Oct. 11, 2024, the World Health Organization (WHO) reported that a total of 58 cases of Marburg…
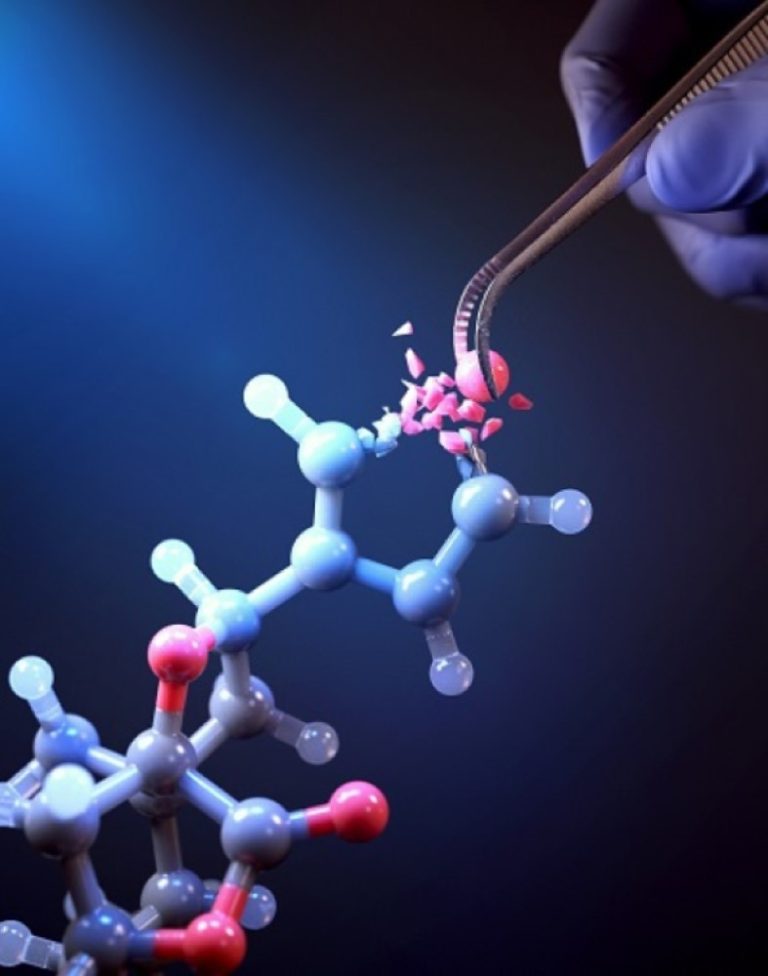
On Oct. 11, 2024, scientists at the Korea Advanced Institute of Science and Technology (KAIST) announced they had…

On Oct. 11, 2024, researchers in China published a study that provided details about a new wetland virus….

On Oct. 10, 2024, health officials from the U.S. Centers for Disease Control and Prevention announced they had…
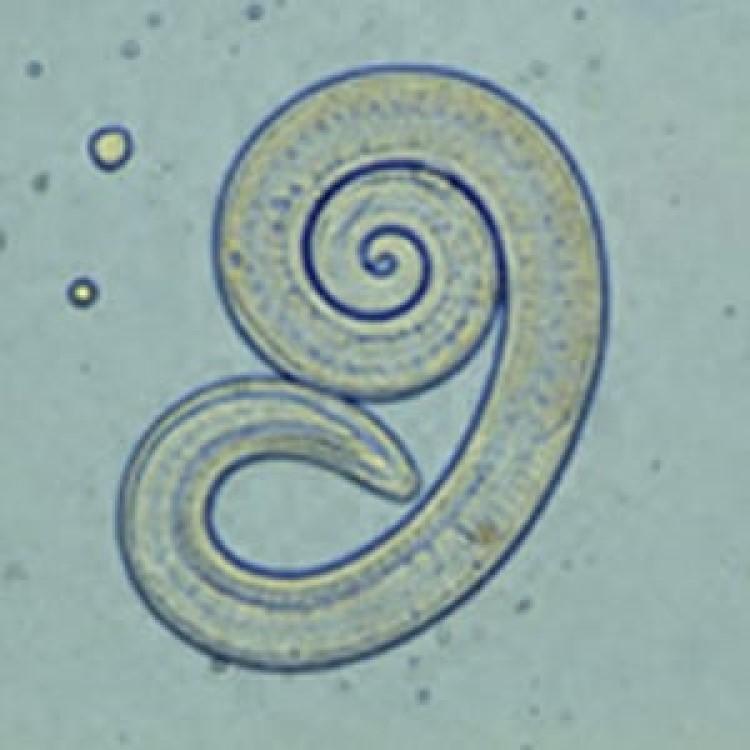
On Oct. 10, 2024, the U.S. Centers for Disease Control and Prevention announced that a 2023 presumed outbreak…

On Oct. 10, 2024, a report by the World Health Organization (WHO) found that vaccines against 24 pathogens…
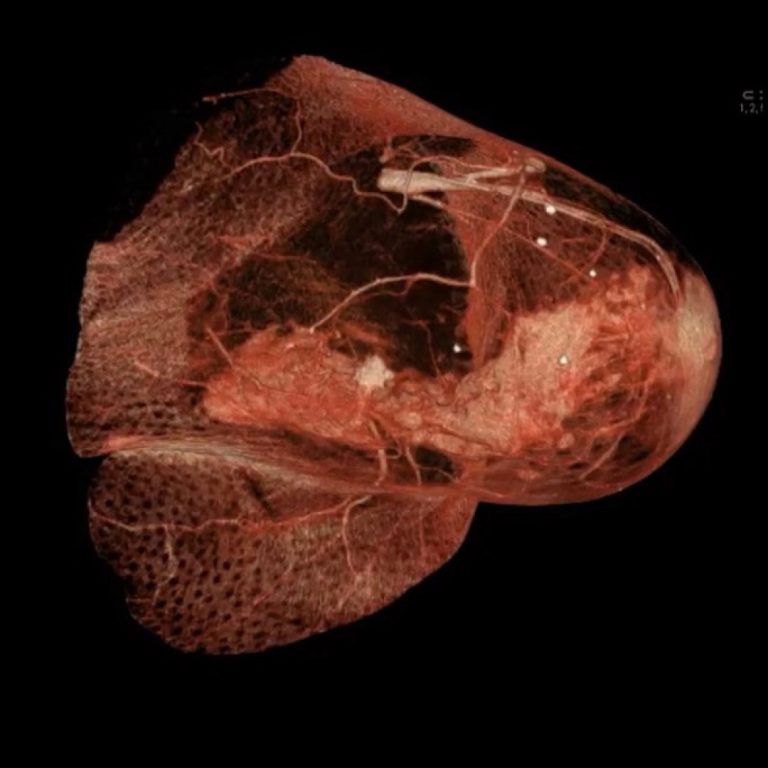
On Oct. 10, 2024, Genentech announced today that the U.S. Food and Drug Administration (FDA) approved ItovebiTM (inavolisib),…

On Oct. 9, 2024, a study led by Johns Hopkins Medicine researchers concluded that commonly used ways of…
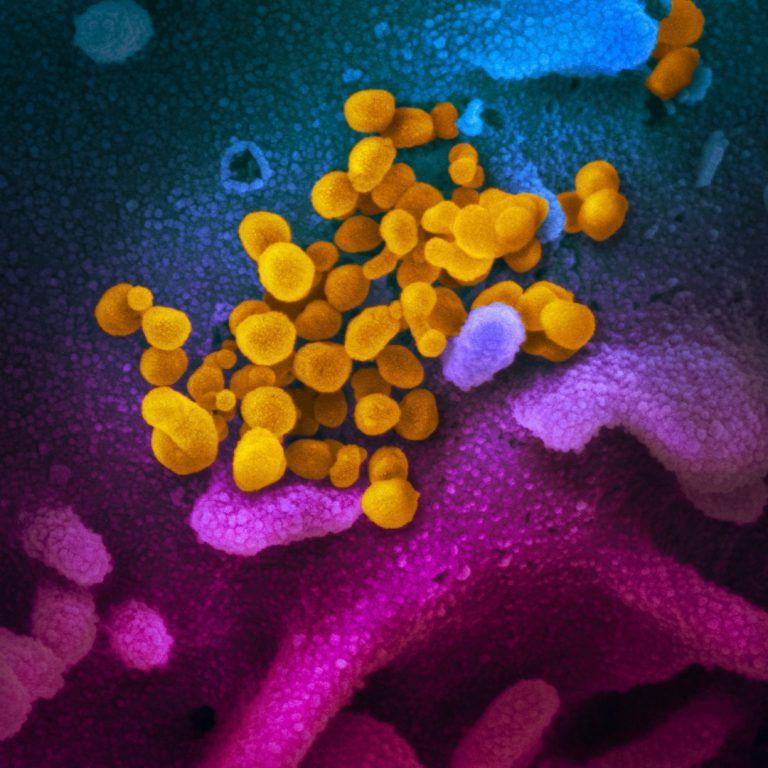
On Oct. 9, 2024, researchers from Brigham and Women’s Hospital found that people with wide-ranging long COVID symptoms…