U.S. Supreme Court upheld 1962 drug effectiveness law and endorsed FDA action to control products by regulation
On Jun. 18, 1973, the U.S. Supreme Court upheld the 1962 drug effectiveness law and endorsed Food and…

On Jun. 18, 1973, the U.S. Supreme Court upheld the 1962 drug effectiveness law and endorsed Food and…

On Jun. 9, 1973 Secretariat won the Belmont Stakes by 31 lengths and a time of 2:24 flat,…

On May 19, 1973 Secretariat won the Preakness Stakes in a record setting time of 1:53, a stakes…

On May 5, 1973 Secretariat won the Kentucky Derby in a record setting time of 1:59 2/5, a…

In 1973, the American Chemical Society announced that the Priestley Medal was awarded to Harold C. Urey “to…

In 1973, Satoshi Ōmura discovered the extraordinary microorganism that produces the avermectins (from which ivermectin is derived) that…
In 1973, certification in medical oncology and gynecologic oncology was first offered.

In 1973, Bristol-Myers introduced several early medicines, beginning with BLENOXANE (bleomycin sulfate) for squamous cell cancers, head and…
In 1973, American scientists Michael E. Phelps and Edward J. Hoffman developed position emission tomography (PET) scans, a…

In 1973, the Ohio State University Cancer Center (OSUCCC) was established in Columbus. The patient care arm of…

In 1973, the Norris Cancer Center was designated a National Cancer Institute (NCI) Comprehensive Cancer Center, one of…

In 1973, Oregon University Hospital was created through the merger of Multnomah County Hospital, Medical School Hospital and…

In 1973, Penn Medicine’s Cancer Center was formally established by a dedicated group of cancer specialists committed to…

In 1973, Stanford Medicine researchers demonstrated the first expression of a foreign gene implanted in bacteria by recombinant…

In 1973, Stephen C. Jacobsen at University of Utah, developed the Utah Artificial Arm in 1973, the world’s…

In 1973, The National surveillance of Reye syndrome began in 1973 when the Center for Disease Control and…

In 1973, the University of Wisconsin Carbone Cancer Center received National Cancer Center designation from the National Cancer…

In 1973, the Office of Biosafety, now known as the Division of Laboratory Systems (DLS) was established at…

In 1973, the University of California, San Diego Regional Burn Center opened, serving San Diego and Imperial counties….

In 1973, Dr. Boone Chunprapah at the Cook County Hospital I Chicago became the first doctor to successfully…

In 1973, the Norris Comprehensive Cancer Center, now the University of Southern California Kenneth Norris Jr. Cancer Center…

In 1973, UChicago established the University of Chicago Comprehensive Cancer Center (UCCCC), receiving its initial designation as a…

In 1973, clinicians at the Boston Hospital for Women, now part of Brigham and Womenメs, developed noninvasive fetal…

In 1973, The Johns Hopkins Cancer Center, now known as the Sidney Kimmel Cancer Center (SKCCC) was founded….
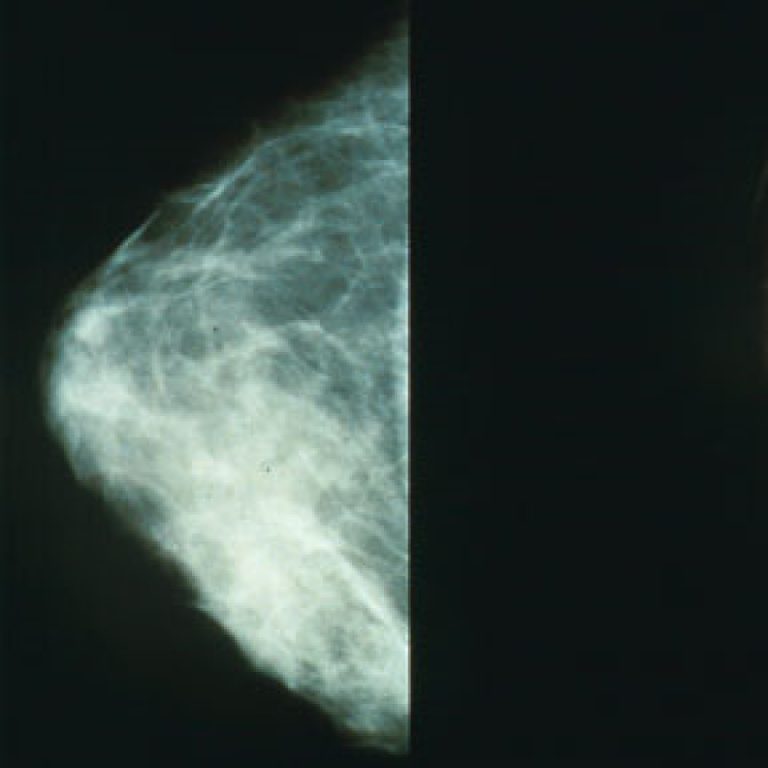
In 1973, Karmanos Cancer Institute researcher Herbert Soule, Ph.D. developed the MCF-7 human breast cancer cell line, which…

In 1973, the publication “Biohazards in Biological Research,” was edited by S. Hellman, M. Oxman, and R. Pollack. …

In 1973, the Michigan Cancer Foundation’s cancer registry was invited to join the National Cancer Institute’s (NCI) Surveillance,…

In 1973, Senator Norris Cotton secured a second federal grant of $500,000 to support cancer research at Geisel…

In 1973, The Surveillance, Epidemiology, and End Results (SEER) Program was established. The SEER Program was a sequel…

In 1973, The trustees of Mary Hitchcock Memorial Hospital allocated funds to help build a two-story addition to…