University of Arizona Cancer Center was designated by the NCI as an official cancer research and treatment center
In 1978, The University of Arizona Cancer Center (UACC) received its first National Cancer Institute (NCI) Support Grant…

In 1978, The University of Arizona Cancer Center (UACC) received its first National Cancer Institute (NCI) Support Grant…

In 1978, the University of California, San Diego Cancer Center was founded as one of just 45 National…

In 1978, University of California, Berkeley (UC-Berkeley) scientist Choh Hao Li discovers Beta-endorphin, a substance produced in the…

In 1978, The Barbara Davis Center for Childhood Diabetes (BDC) was founded by Marvin Davis, former chair of…

On Nov. 23, 1977, the Saccharin Study and Labeling Act was enacted by the U.S. Congress to stop…
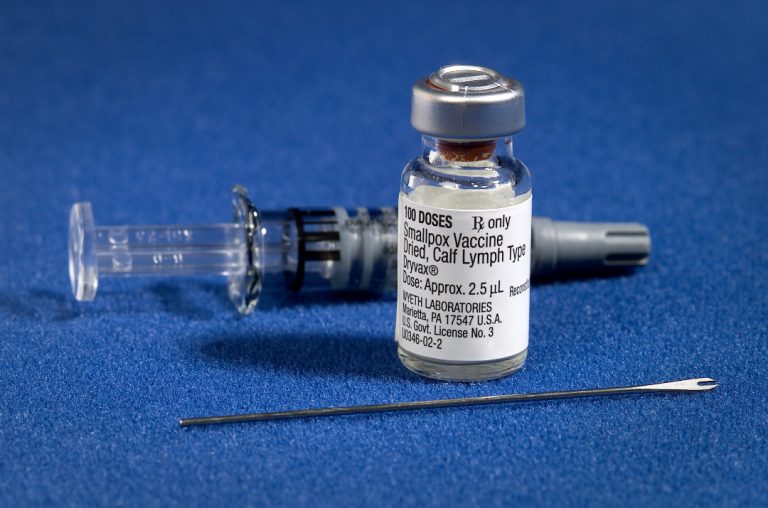
On Oct. 30, 1977, Ali Maow Maalin, a hospital cook in Merca, Somalia, was diagnosed with smallpox by…

On Aug. 31, 1977, in recognition of the seriousness of lupus and America’s commitment to its control, the…

On Jul. 29, 1977, Arthur Canfield Upton, M.D. became the eighth director of the National Cancer Institute (NCI)…

On May 12, 1977, the Program for the Introduction and Adaptation of Contraceptive Technology (PIACT) was founded by…

On Apr. 6, 1977, the Department of Health, Education, and Welfare (now Department of Health and Human Services)…

On Apr. 4, 1977, Donald Kennedy, Ph.D., became Commissioner of the U.S. Food and Drug Administration (FDA). Kennedy,…

In 1977, the Priestley Medal was awarded to Henry Gilman by the American Chemical Society “to recognize distinguished…
On Mar. 7, 1977, the U.S. Food and Drug Administration approved Bristol-Myers’ BICNU (carmustine), for the treatment of…

In 1977, the first national cancer patient education program (I Can Cope) was founded by the National Cancer…

In 1977, the National Cancer Institute (NCI) established the first electronic registry of cancer clinical trials (CLINPROT). This…

In 1977, Dr. Henry Friesen of McGill University discovered the hormone prolactin and defined its role as a…
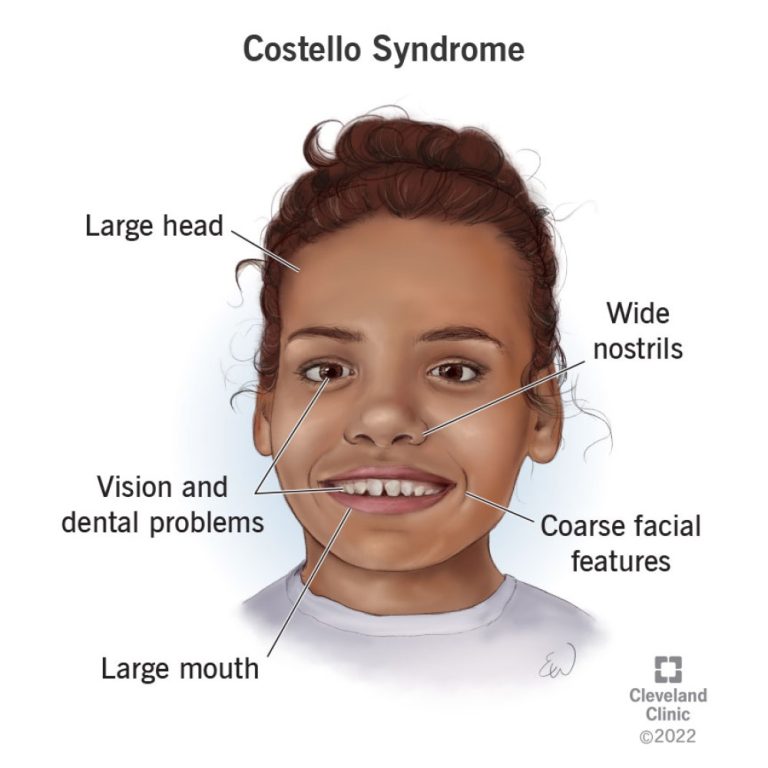
In 1977, Dr. Jack Costello, a geneticist in New Zealand, first identified a genetic disorder, now known as…

In 1977, St.ᅠ Jude Children’s Research Hospital received National Cancer Institute designation. St.ᅠJude Children’s Research Hospital opened it’s…

In 1977, the International Board for Plant Genetic Resources (IBPGR) having inherited from the Food and Agriculture Organization’s…

In 1977, the U.S. Food and Drug Administration (FDA) established the Bioresearch Monitoring Program (BiMo) to develop cross-center…

In 1977, Joseph A. Califano, Jr., Secretary of the Dept of Health, Education, and Welfare (later Health and…

In 1977, Rosalyn Yalow won a share of the Nobel Prize in Physiology or Medicine for the development…

In 1977, the multiple research programs that had developed were formally drawn together into the Research Institute of…

In 1977, Charles C. Edwards, formerly the nation’s top government health official, was named president and CEO of…

In 1977, Stanford Research Institute changed its name to SRI International. Stanford Research Institute, now known as the…

In 1977, the original Naval Medical Center tower was designated a historical landmark and entered into the Registry…
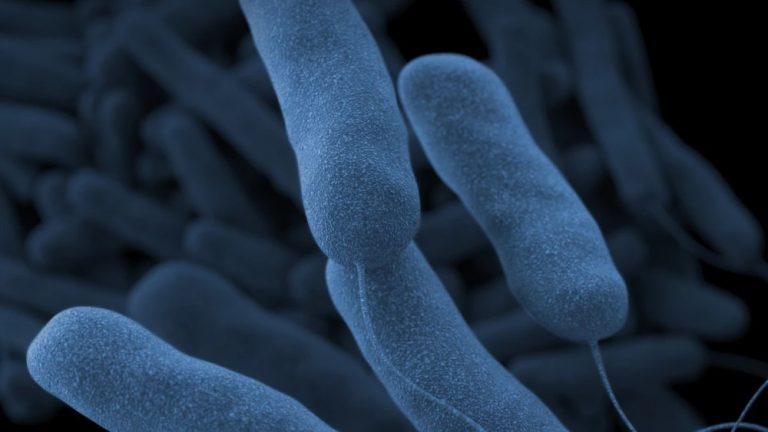
In 1977, the U.S. Centers for Disease Control and Prevention (CDC) isolated Legionella pneumophila, which caused a deadly…

In 1977, Eugene Goldwasser published Purification of Human Erythropoietin (EPO) in the Journal of Biological Chemistry. Goldwasser spent…

In 1977, Dana-Farber (DF) received National Cancer Institute (NCI) Comprehensive Cancer Center designation one of the first in…
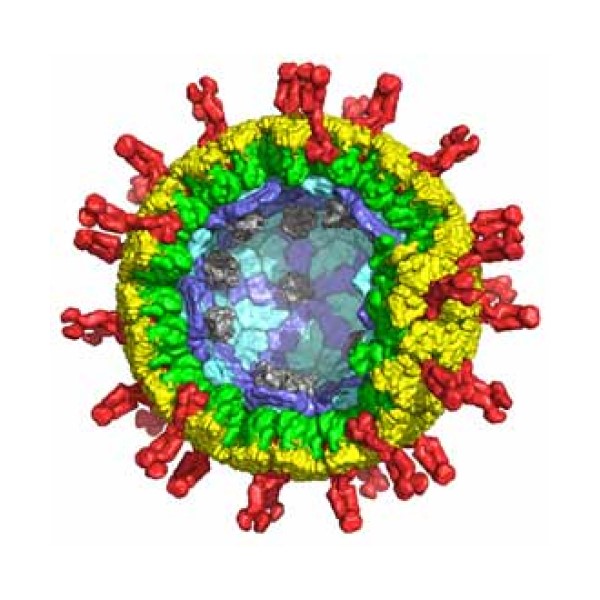
In 1977, Harvard Medical School researcher Stephen C. Harrison first determined the structure of an intact virus particle,…