Gladstone Institutes was founded
In 1979, the Gladstone Institutes was founded from an endowment from J. David Gladstone, a self-made man who…

In 1979, the Gladstone Institutes was founded from an endowment from J. David Gladstone, a self-made man who…

In 1979, Joan Steitz discovered snRNPs, RNA-protein complexes in the cell’s nucleus that perform a crucial step in…
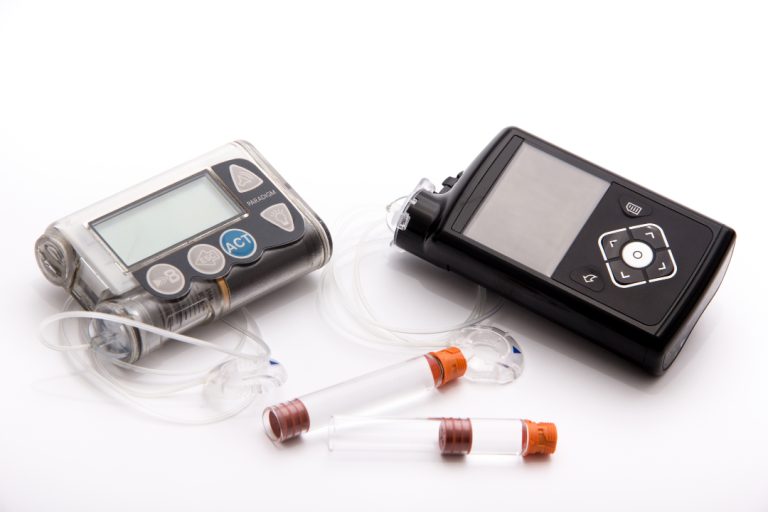
In 1979, Dr. Robert Sherwin and Dr. William Tamborlane from Yale University announced they had developed an insulin…

In 1979, Medtronic established a Heart Valves division and introduced the Medtronic Hall mechanical heart valve. This prosthetic…

In 1979, Dr. Norman H. Cromwell of the Department of Chemistry, University of Nebraska-Lincoln, became acting director of…

In 1979, Centocor was founded – one of the nation’s first biotechnology companies best known for developing Remicade…

In 1979, the University of Pennsylvania’s Institute on Aging (IOA) was created to improve the health of the…

In 1979, The National Association for Biomedical Research (NABR) was founded providing the unified voice for the scientific…
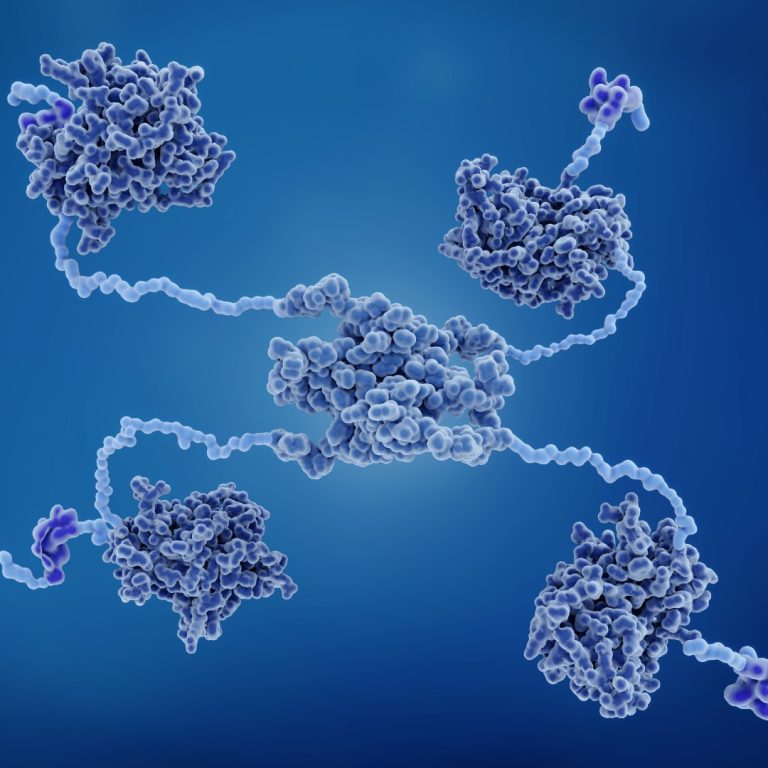
In 1979, the most frequently mutated gene in human cancer, p53, was identified by six groups of researchers…

In 1979, early concern over the dangers of recombinant DNA has waned and the National Institutes of Health…
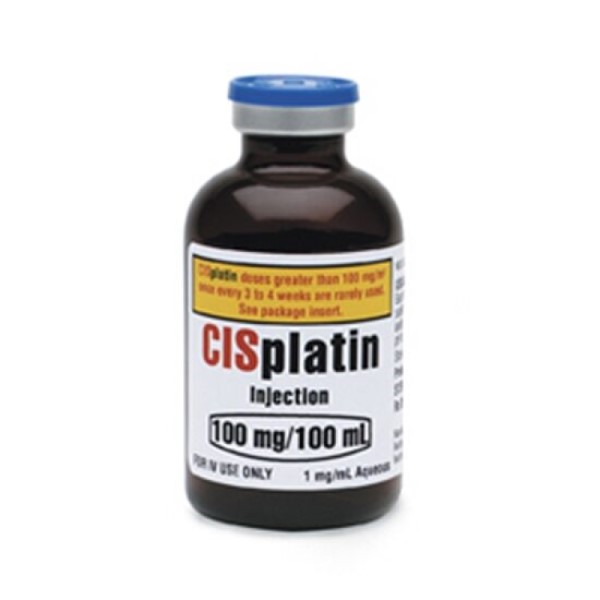
On Dec. 19, 1978, the U.S. Food and Drug Administration (FDA) approved cisplatin (Platinol) for use in combination…

On Nov. 9, 1978, the President Jimmy Carter signed the Community Mental Health Centers Act (P.L. 95-622) amending…

On Nov. 9, 1978, the first human testing of a biological therapy was conducted (alpha-interferon). The Cancer Research…

On Oct. 3, 1978, the University of Washington Health Sciences Building was renamed the Magnuson Health Sciences Center…
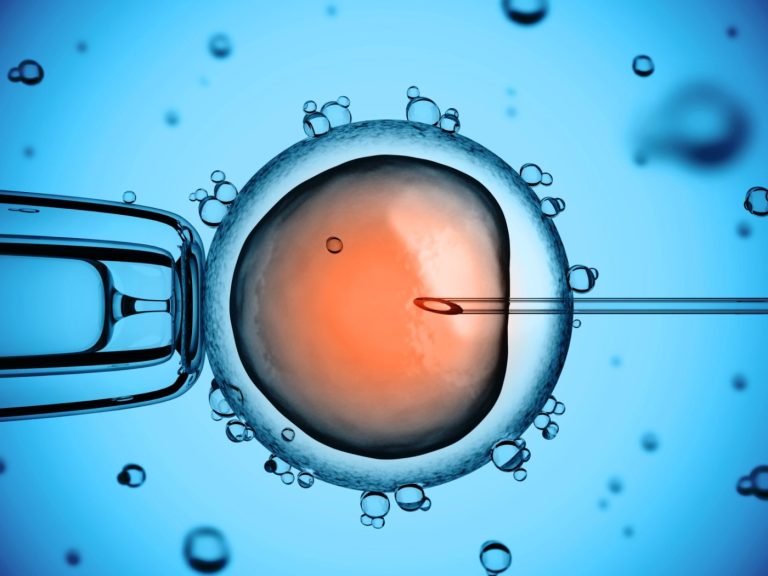
On Jul. 25, 1978, the first test-tube baby was born in the United Kingdom. Louise Joy Brown is…

On Jul. 13, 1978, Ontario researchers published the results of a study led by Dr. Henry Barnett that…
In May 1978, the U.S. Centers for Disease Control and Prevention (CDC) reported a patient was first diagnosed…

In 1978, The Priestley Medal was awarded to Melvin Calvin by the American Chemical Society “to recognize distinguished…
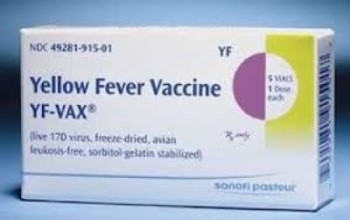
On Jan. 3, 1978, the Yellow fever vaccine (YF-Vax by Connaught) was licensed in the U.S. The Yellow…

On Jan. 3, 1978, the U.S. Food and Drug Administration (FDA) licensed the Monovalent group A (Menomune-A by…

In August 1978, molecular biologist Lydia Villa-Komaroff was lead author of a paper demonstrating that bacteria could produce…

In 1978, Squibb formed ConvaTec as a separate division to develop adhesive skin barriers and products that could…

In 1978, Johnsonᅠ & ᅠJohnson announced plans to build their new World Headquarters in New Brunswick, New Jersey….

In 1978, Dr. Shannon Lucid, a researcher in the Biomembrane Research Laboratory, was selected by NASA as one…

In 1978, metastatic cells were shown to arise from pre-existing subpopulations of cells in primary tumors.

In 1978, the Carbone Cancer Center Clinical Sciences Center was completed in January adding over 70,000 square feet…

In 1978, Carl Woese, an American microbiologist defined Archaea as a new domain based upon genetic relationships that…
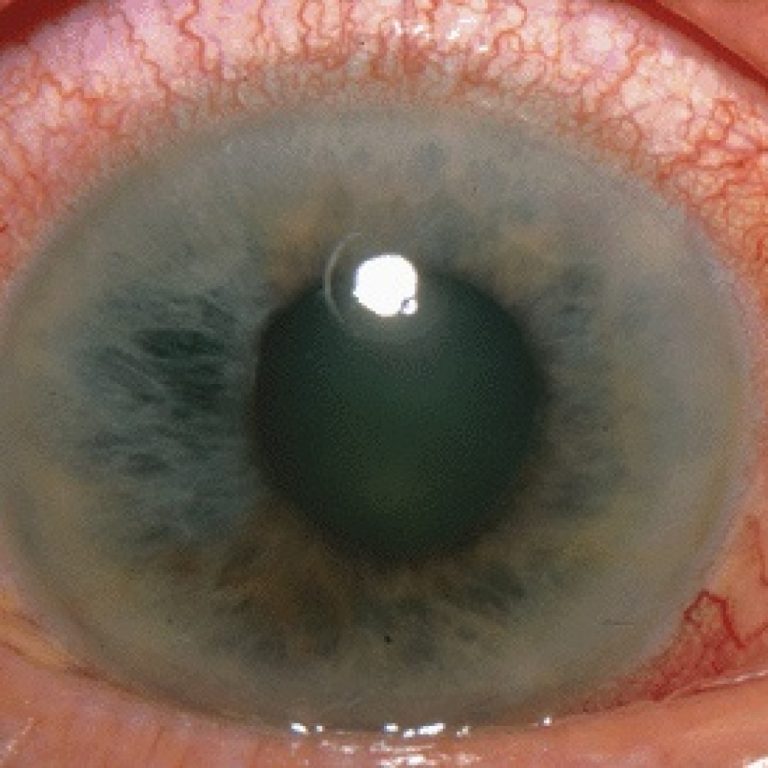
In 1978, Drs. Shaffer, Hoskins and Hetherington founded the Glaucoma Research Foundation (GRF), now America’s oldest and most…

In 1978, The University of Arizona Cancer Center (UACC) received its first National Cancer Institute (NCI) Support Grant…

In 1978, the University of California, San Diego Cancer Center was founded as one of just 45 National…