EC expanded indication for Veklury (Remdesivir) for treatment of adults not on supplemental oxygen and high risk for COVID-19 progression
On Oct. 21, 2021, Gilead Sciences announced that the European Commission (EC) had approved a variation to the…

On Oct. 21, 2021, Gilead Sciences announced that the European Commission (EC) had approved a variation to the…

On Dec. 21, 2021, the United States Department of Agriculture’s (USDA) National Veterinary Services Laboratories announced confirmation of…

On Dec. 20, 2021, Oragenics announced it had extended a licensing and collaboration agreement with the National Research…

On Dec. 16, 2021, NIH scientists announced that to counter future coronavirus outbreaks, the global scientific and medical…

On Dec. 16, 2021, Merck and Ridgeback Biotherapeutics announced the New England Journal of Medicine had published findings…

On Dec. 15, 2021, Twist Bioscience announced that synthetic RNA positive controls were available for the SARS-CoV-2 Omicron…

On Dec. 15, 2021, Johnson & Johnson announced that the Committee for Medicinal Products for Human Use (CHMP)…

On Dec. 14, 2021, NRx Pharmaceuticals provided a new safety update on ZYESAMIᆴ (aviptadil), which was being tested…

On Dec. 12, 2021, the U.S. Department of Health and Human Services (HHS) announced the distribution of approximately…

One year ago, on Dec. 14, 2020, the Military Health System began administering the first COVID-19 vaccine shots….

On Dec. 13, 2021, the Coalition for Epidemic Preparedness Innovations, and Affinivax announced a partnership to advance the…

On Dec. 13, 2021, researchers from the University of Oxford analysed the impact of the Omicron COVID-19 variant…

On Dec. 9, 2021, Pfizer and BioNTech announced that the U.S. Food and Drug Administration (FDA) had expanded…

On Dec. 8, 2021, the U.S. Food and Drug Administration issued an emergency use authorization for AstraZenecaメs Evusheld…

On Dec. 7, 2021, the Centers for Disease Control and Prevention announced that it had awarded $22 million…

On Dec. 7, 2021, Arbutus Biopharma, X-Chem, and Proteros announced that Arbutus had identified several molecules that inhibit…

On Dec. 6, 2021, Roche announced that the European Medicines Agencyメs Committee for Medicinal Products for Human Use…

On Dec. 6, 2021, the World Health Organization Guideline Development Group of international experts announced in the BMJ…

On Dec. 6, 2021, the USAID announced the foundation of a new whole-of-government effort, the Initiative for Global…

On Dec. 5, 2021, Johnson & Johnson announced preliminary results from an independent study, including a subset of…

On Dec. 3, 2021, Genomics England has announced its vision for a pilot research project to examine the…

On Dec. 3, 2021, Gavi, the Vaccine Alliance, approved a new malaria vaccination programme to support the introduction,…

On Dec. 3, 2021, Roche and TIB Molbiol announced they had added three additional Research Use Only test…

On Dec. 2, 2021, researchers at the National Eye Institute (NEI) announced they had identified, isolated, and characterized…

On Dec. 2, 2021, National Resilience and Harvard University announed they had established a five-year research and development…

On Dec. 1, 2021, Fulgent Genetics confirmed that the Companyメs RT-PCR test for SARS-CoV-2, the virus that causes…
On Nov. 30, 2021, LabCorp announced that it had begun offering observed self-collection for COVID-19 PCR testing in…

On Nov. 29, 2021, Thermo Fisher Scientific confirmed that its polymerase chain reaction (PCR) TaqPath COVID-19 Combo Kit…
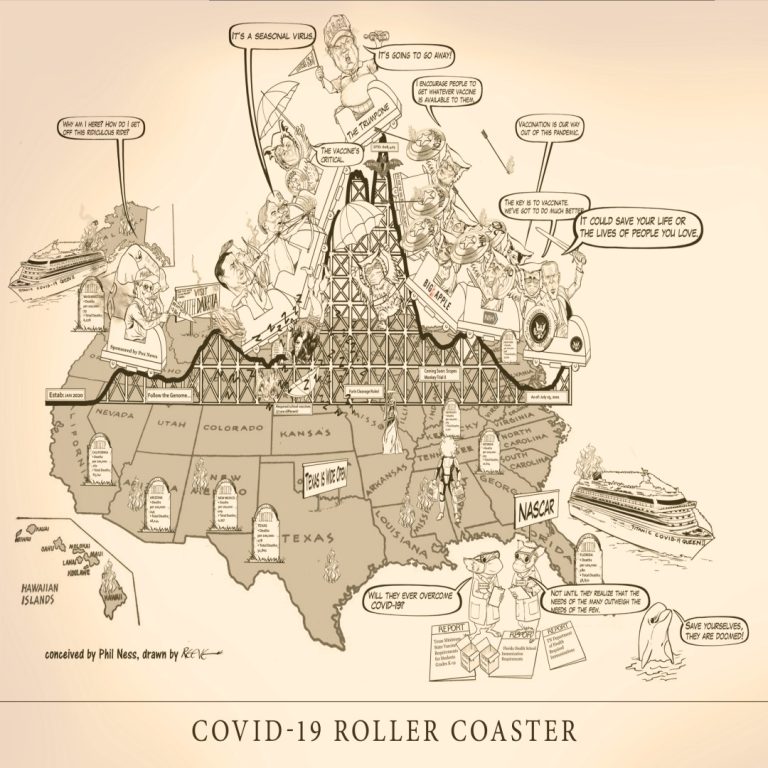
The COVID-19 Roller Coaster is a wild ride, strap yourself in and hold on… Cast of Characters: Senator…

On Nov. 26, 2021, the World Health Organization announced that the Technical Advisory Group on SARS-CoV-2 Virus Evolution…