Raw milk containing H5N1 can infect mice, while lab-based heat treatments greatly reduce the virus
On May 24, 2024, a team led by University of Wisconsin–Madison scientists reported that consuming raw cow’s milk…

On May 24, 2024, a team led by University of Wisconsin–Madison scientists reported that consuming raw cow’s milk…

On May 23, 2024, the U.S. Centers for Disease Control and Prevention (CDC) reported that 109 people from…
On May 22, 2024, the World Health Organization (WHO) announced that the first confirmed case of human infection…

On May 20, 2024, the Michigan Department of Agriculture and Rural Development (MDARD) announced the detection of highly…

On May 10, 2024, Novavax announced that it had entered into a co-exclusive licensing agreement with Sanofi. The…

On Apr. 26, 2024, the Colorado Dept. of Agriculture reported that the U.S. Department of Agriculture’s (USDA) National…
On Apr. 23, 2024, the U.S. Food and Drug Administration (FDA) announced that nearly all (99%) of the…

On Apr. 21, 2024, United States Department of Agriculture’s (USDA) APHIS National Veterinary Services Laboratories announced it had…

On Apr. 12, 2024, the U.S. Centers for Disease Control and Prevention (CDC) announced that Vietnam had reported…

On Apr. 5, 2024, the U.S. Centers for Disease Control and Prevention (CDC) announced that Texas had reported…

On Apr. 2, 2024, the U.S. Department of Agriculture (USDA) confirmed the detection of highly pathogenic avian influenza…

On Apr. 1, 2024, the U.S. Department of Agriculture (USDA) confirmed the detection of highly pathogenic avian influenza…

On Mar. 29, 2024, the U.S. Centers for Disease Control and Prevention (CDC) reported an infection with an…

On Mar. 28, 2024, the Idaho State Department of Agriculture (ISDA) announced it had identified a highly pathogenic…

On Mar. 25, 2024, immediately following the first detection of H5N1 in dairy cattle in the Texas panhandle…

On Mar. 20, 2024, the Minnesota Board of Health announced that a Stevens County goat kid (juvenile goat)…

On Mar. 11, 2024, the British Antarctic Survey reported that penguins on the sub-Antarctic Islands of South Georgia…

On Mar. 8, 2024, the U.S. Food and Drug Administration’s (FDA) Vaccines and Related Biological Products Advisory Committee…

On Mar. 5, 2024, the World Health Organization (WHO) announced that in February 2024, Austria, Denmark, Germany, Sweden…
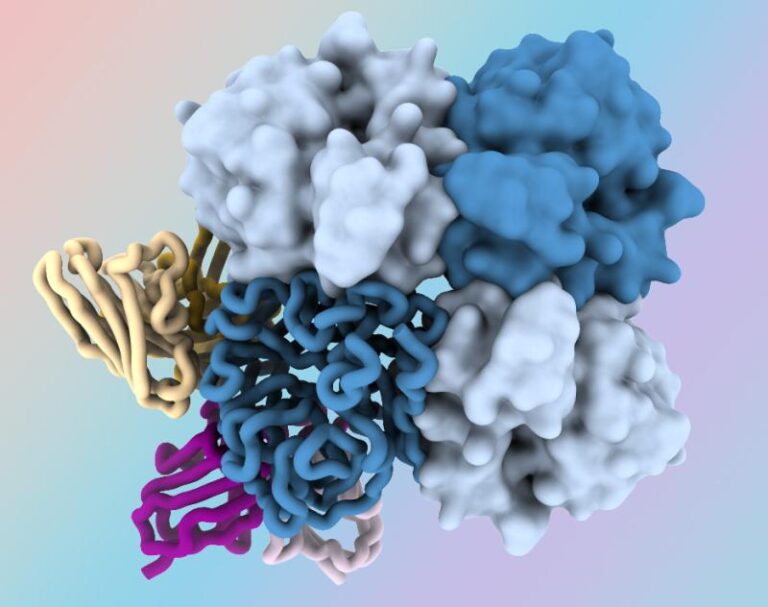
On Mar. 1, 2024, researchers at the National Institutes of Health (NIH) announced they had identified antibodies targeting…

On Feb. 20, 2024, the U.S. Department of Agriculture (USDA) confirmed the presence of highly pathogenic avian influenza…

On Feb. 12, 2024, the U.S. Centers for Disease Control and Prevention (CDC) announced that Cambodia had reported…

On Jan. 31, 2024, a U.S. Centers for Disease Control and Prevention (CDC) co-authored study published in Influenza…

On Jan. 27, 2024, the National Health Commission of the People’s Republic of China notified the World Health…

On Jan. 22, 2024, the University of California, Davis announced the first reported outbreak of high pathogenicity H5N1…

On Jan. 9, 2024, biologists in Alaska have confirmed the first case of a polar bear dying after…

On Jan. 4, 2024, France announced it had detected bird flu on a duck farm in the Vendee…

On Dec. 18, 2023, the Novo Nordisk Foundation announced it was committing up to DKK 1.8 billion (USD…

On Dec. 18, 2023, the U.S. Centers for Disease Control and Prevention (CDC) announced a study that showed…
On Dec. 1, 2023, the World Health Organization (WHO) announced that the International Health Regulations National Focal Point…