USDA confirmed highly pathogenic Avian influenza in a commercial poultry flock in Delaware
On Feb. 23, 2022, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) confirmed…

On Feb. 23, 2022, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) confirmed…

On Feb. 19, 2022, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) confirmed…

On Feb. 18, 2022, the U.S. Centers for Disease Control and Prevention (CDC) released the 2022 recommended immunization…

On Feb. 14, 2022, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) confirmed…

On Feb. 14, 2022, The U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspective Service (APHIS) announced…

On Feb. 9, 2022, the Indiana Department of Health confirmed that a case of H5N1 highly pathogenic avian…
On Feb. 2, 2022, NIAID scientists announced the new Pandemic Preparedness Plan aimed to support critical basic and…

On Jan. 25, 2022, Cepheid announced that Health Canada had issued Cepheid a medical device license for Xpert…

On Jan. 14, 2022, the U.S. Department of Agriculture’s (USDA) National Veterinary Services Laboratories confirmed a highly pathogenic…
On Jan. 13, 2022, the Centers for Disease Control and Prevention announced a study published in the journal…

On Dec. 5, 2021, Roche announced that its planned to launch the SARS-CoV-2 & Flu A/B Rapid Antigen…
On Oct. 27, 2021, Hologic announced that its Aptima SARS-CoV-2/Flu Assay was available for the simultaneous detection and…

On Oct. 22, 2021, the U.S. Centers for Disease Control and Prevention (CDC) reported two new U.S. human…
On Oct. 7, 2021, PerkinElmer announced that the U.S. Food and Drug Administration (FDA) had issued Emergency Use…

On Oct. 7, 2021, Vaxart announced that a Duke University-led study published in bioRxiv showed that Vaxart’s (investigational…
On Oct. 1, 2021, LabCorp announced that it had received Emergency Use Authorization (EUA) from the U.S. Food…

On Sept. 27, 2021, Pfizer announced that the first participants have been dosed in a Phase 1 clinical…

On Sept. 23, 2021, Novavax announced publication of complete results from a pivotal Phase 3 clinical trial of…

On Sept. 8, 2021, Novavax announced enrollment of the first participants in a Phase 1/2 study to evaluate…
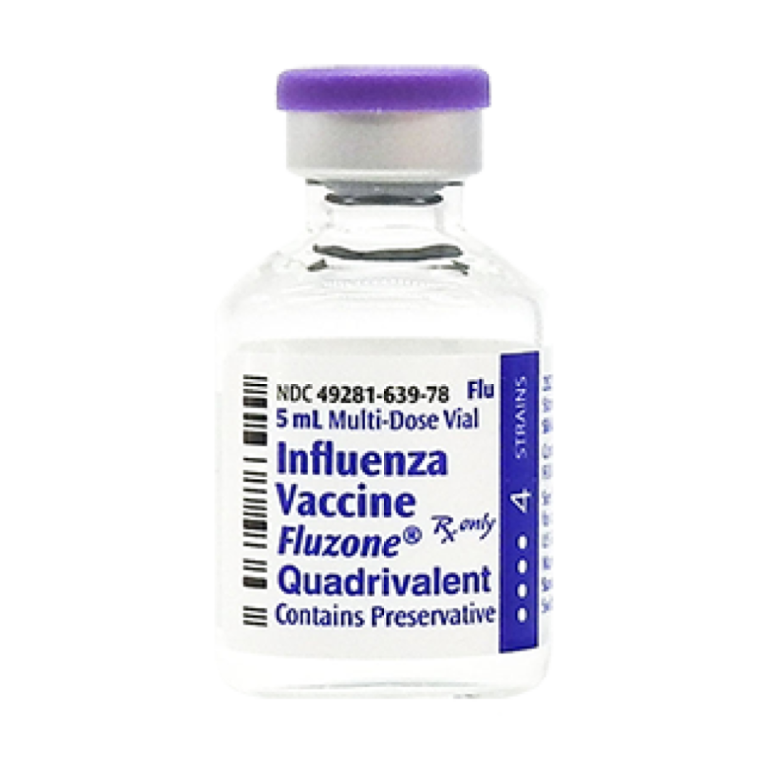
On Jun. 17, 2021, the U.S. Food and Drug Administration (FDA) Center for Biologics Evaluation and Research granted…

On Jun. 14, 2021, Novavax announced data from the first co-administration study of a SARS-CoV-2 vaccine candidate [Novavax,…
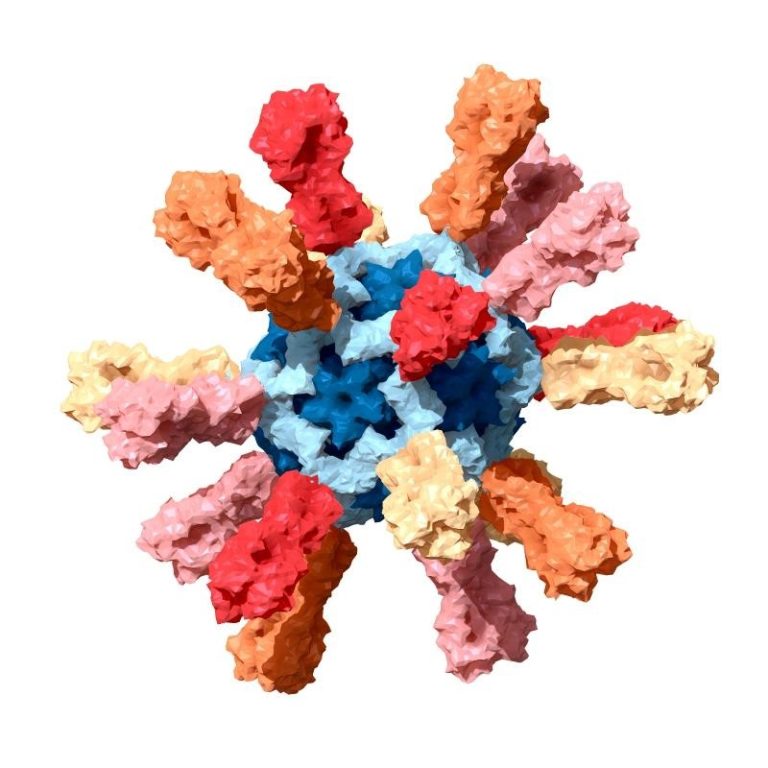
On Jun. 1, 2021, the National Institutes of Health (NIH) announced the first-in-human, Phase 1 trial assessing the…

On May 10, 2021, Novavax announced data from a preclinical study of the company’s combination quadrivalent seasonal flu…

On Apr. 15, 2021, the National Institute of Allergy and Infectious Diseases (NIAID) announced it had had established…

On Apr. 8, 2021, Oxford University announced that favipiravir was to be investigated in the UK as part…

On Mar. 30, 2021, BD (Becton, Dickinson) announced the U.S. Food and Drug Administration (FDA) granted emergency use…

On Mar. 5, 2021, Abbott announced the U.S. Food and Drug Administration’s (FDA) Emergency Use Authorization (EUA) for…

On Feb. 20, 2021, Russian authorities reported the detection of influenza A(H5N8) virus infection in seven poultry workers…

On Feb. 19, 2021, Luminex announced that it had received $11.3 million in funding from the Biomedical Advanced…

On Feb. 3, 2021, an experimental single-dose, intranasal influenza vaccine, was safe and produced a durable immune response…