BD launched high-throughput molecular combination test for COVID-19 and influenza A/B
On May 26, 2022, BD (Becton, Dickinson) announced a new high-throughput molecular diagnostic combination test for SARS-CoV-2 and…

On May 26, 2022, BD (Becton, Dickinson) announced a new high-throughput molecular diagnostic combination test for SARS-CoV-2 and…

On May 25, 2022, Novavax announced it was participating in a stage of the COVID-19 Vaccine Schedule Combinations…
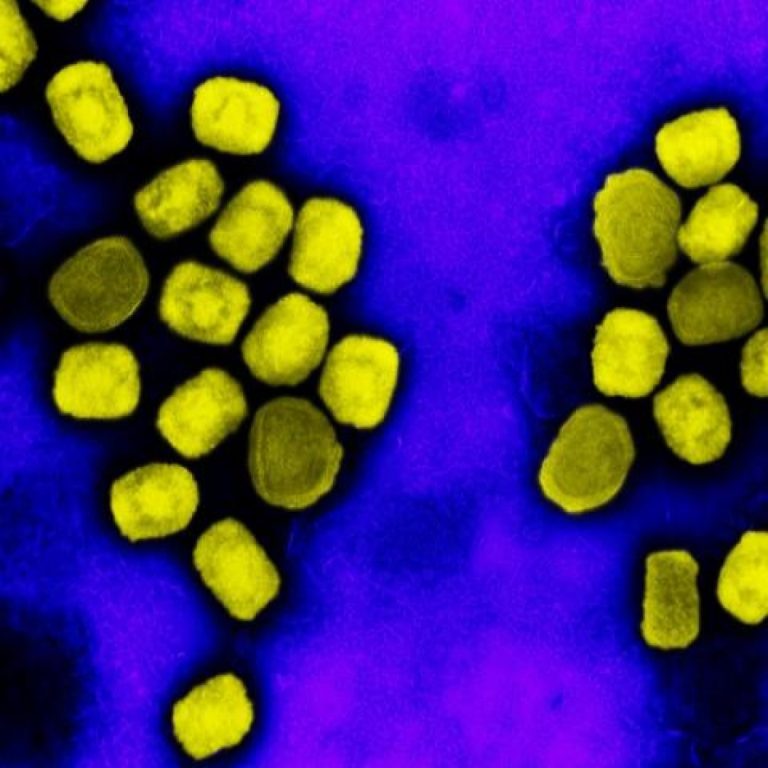
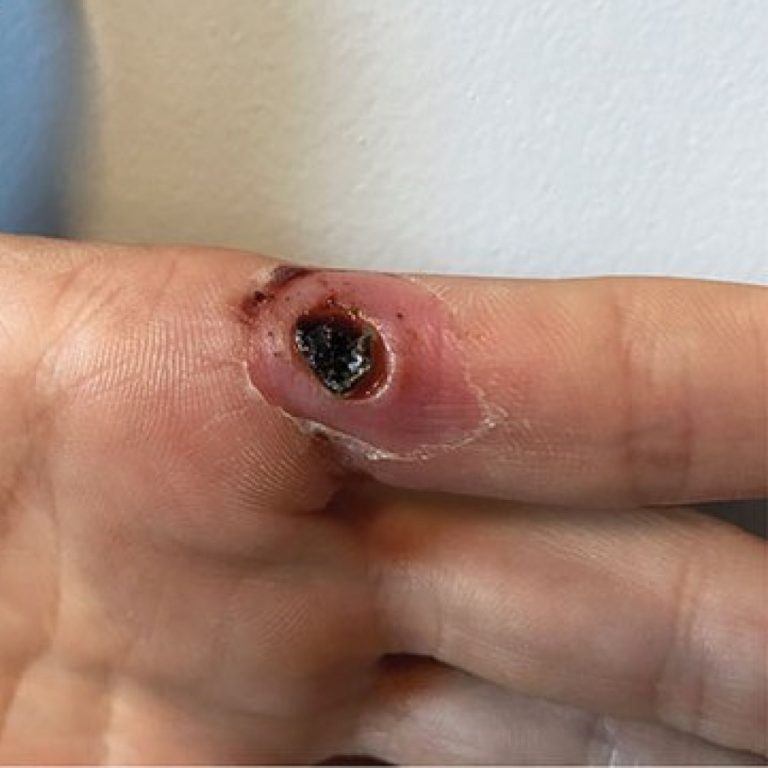
On May 21, 2022, the World Health Organization (WHO) reported that since May 13, 2022, cases of mpox…

On May 20, 2022, Novavax announced the submission of a request to the European Medicines Agency (EMA) to…

On May 18, 2022, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) confirmed…
On May 18, 2022, LabCorp announced the receipt of Emergency Use Authorization (EUA) from the U.S. Food and…
On May 18, 2022, scientists at the Centers for Disease Control and Prevention (CDC) announced that they were…

On May 13, 2022, Novavax announced the submission of a request for emergency use authorization to Taiwan’s Food…

On May 11, 2022, Kaiser Permanente Washington Health Research Institute reported that new treatments for people at high…

On May 6, 2022, Novavax announced the submission of variations to the Australian Therapeutic Goods Agency (TGA) and…

On May 6, 2022, the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service (APHIS) confirmed the…

On May 6, 2022, Novavax announced that deliveries of it’s COVID-19 vaccine continued around the world and the…

On May 1, 2022, the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service confirmed the presence…

On Apr. 30, 2022, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) confirmed…

On Apr. 29, 2022, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) confirmed…

On Apr. 29, 2022, Moderna announced its plan to build a state-of-the-art mRNA vaccine manufacturing facility in Quebec…

On Apr. 29, 2022, Moderna announced that it had submitted for a variation to the conditional marketing authorization…

On Apr. 28, 2022, Centers for Disease Control and Prevention (CDC) reported that a person had tested positive…

On Apr. 28, 2022, Moderna announced that it had submitted a request for emergency use authorization (EUA) for…
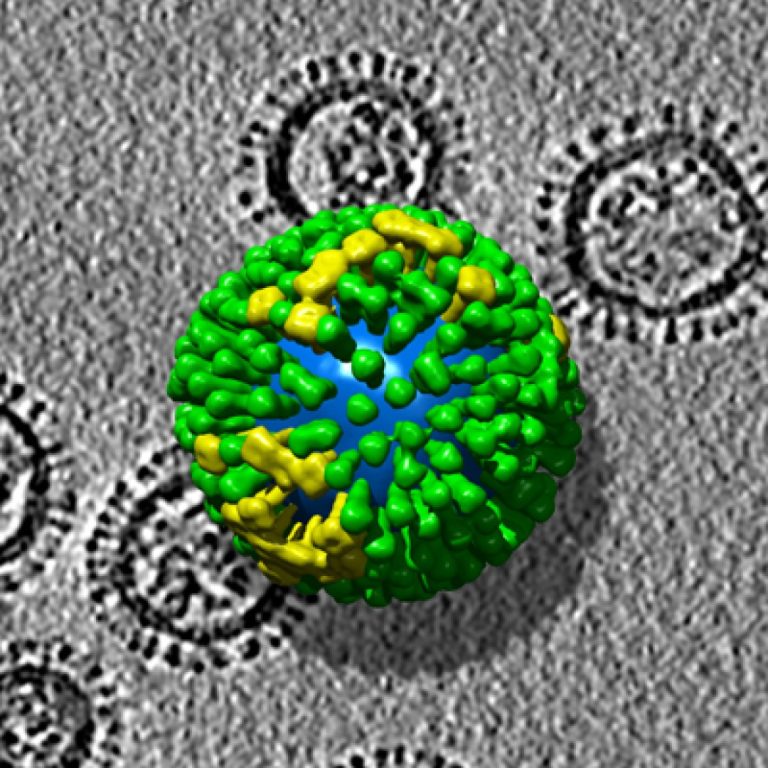
On Apr. 26, 2022, China’s National Health Commission reported a case of human infection with H3N8 avian influenza…

On Apr. 25, 2022, Gilead Sciences announced that the U.S. Food and Drug Administration (FDA) has approved a supplemental…

On Apr. 22, 2022, the World Health Organization (WHO) made a strong recommendation for nirmatrelvir and ritonavir, sold…

On Apr. 22, 2022, Novavax announced initial results from the Phase 1/2 clinical trial of its COVID-Influenza Combination…

On Apr. 21, 2022, CureVac announced preclinical data demonstrating immune responses and protective efficacy of a bivalent second-generation…

On Apr. 20, 2022, Novavax announced initial results from the Phase 1/2 clinical trial of its COVID-Influenza Combination…

On Apr. 20, 2022, Tonix Pharmaceuticals announced the results of a retrospective observational database study in over 50,000…

On Apr. 19, 2022, Novavax announced that its partner, Takeda, received manufacturing and marketing approval from the Japan…

On Apr. 19, 2022, Moderna announced new clinical data on its bivalent COVID-19 booster platform including data on…

On Apr. 18, 2022, the Military Health System reported that active-duty service members who received a COVID-19 vaccine…

On Apr. 16, 2022, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) confirmed…