U.S. CDC endorsed Advisory Committee on Immunization Practices’ recommendation for Novavax COVID-19 vaccine
On Jul. 19, 2022, Novavax announced that the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee…

On Jul. 19, 2022, Novavax announced that the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee…

On Jul. 14, 2022, Moderna announced that Health Canada had approved the use of Moderna’s mRNA COVID-19 vaccine,…

On Jul. 13, 2022, Novavax announced that the European Commission (EC) had approved the expanded conditional marketing authorization…

On Jul. 13, 2022, Novavax announced that its COVID-19 Vaccine, Adjuvanted (NVX-CoV2373) had received emergency use authorization from…

On Jul. 11, 2022, Moderna announced new clinical data on its bivalent Omicron (BA.1) booster candidate, mRNA-1273.214. One…
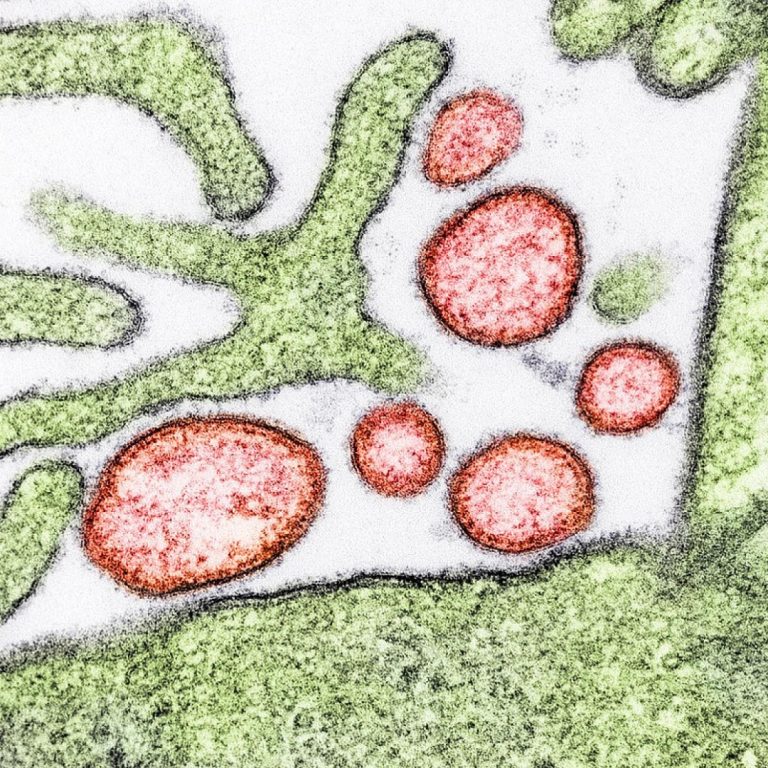
On Jul. 11, 2022, the National Institute of Allergy and Infectious Diseases (NIAID) launched an early-stage clinical trial…

On Jul. 8, 2022, the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service confirmed the state’s…

On Jul. 8, 2022, the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service (APHIS) confirmed the…

On Jul. 7, 2022, Novavax announced that the European Commission had approved a variation to allow SK bioscience…

On Jul. 5, 2022, Novavax announced that the European Commission (EC) had approved the expanded conditional marketing authorization…
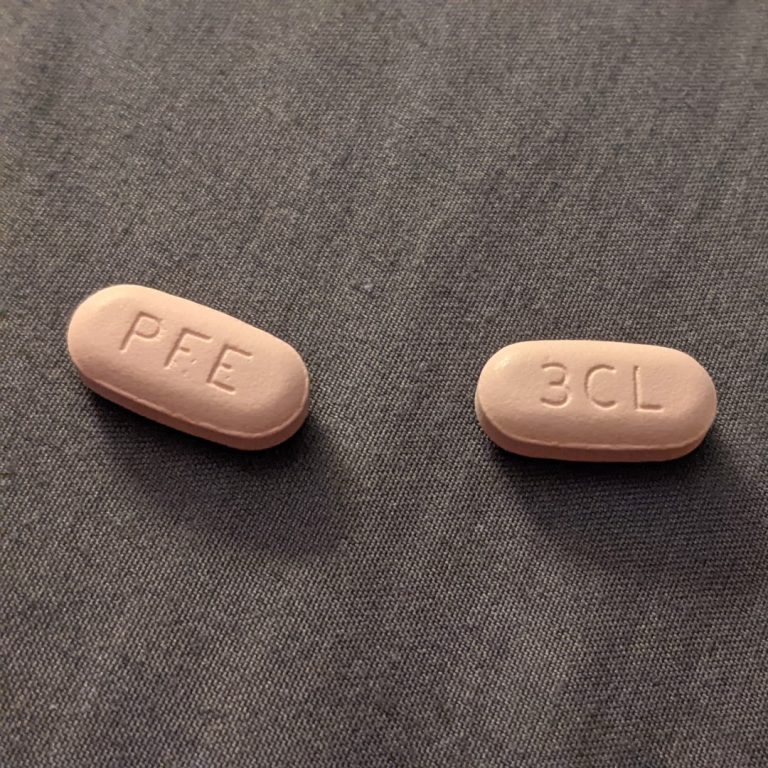
On Jun. 30 2022, Pfizer announced the submission of a New Drug Application (NDA) to the U.S. Food…

On Jun. 30, 2022, BD (Becton, Dickinson) announced that the BD MAX Respiratory Viral Panel (RVP), a new…
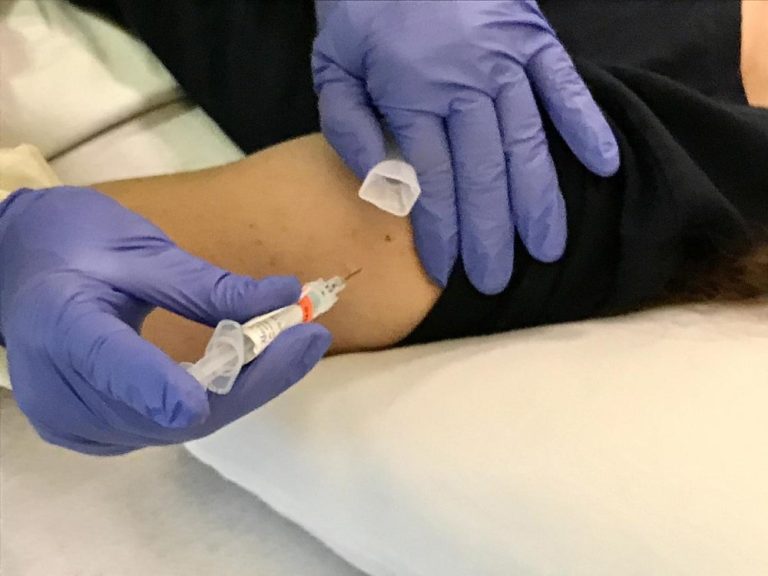
On Jun. 28, 2022, the National Institutes of Health (NIH) announced that a Phase 1 clinical trial of…

On Jun. 23 2022, Novavax announced the filing of a Supplement to a New Drug Submission with Health…

On Jun. 23 2022, Novavax announced that the Nuvaxovid (NVX-CoV2373) COVID-19 vaccine had been recommended for expanded conditional…

On Jun. 23 2022, Novavax announced that the Taiwan Food and Drug Administration had granted emergency use authorization…
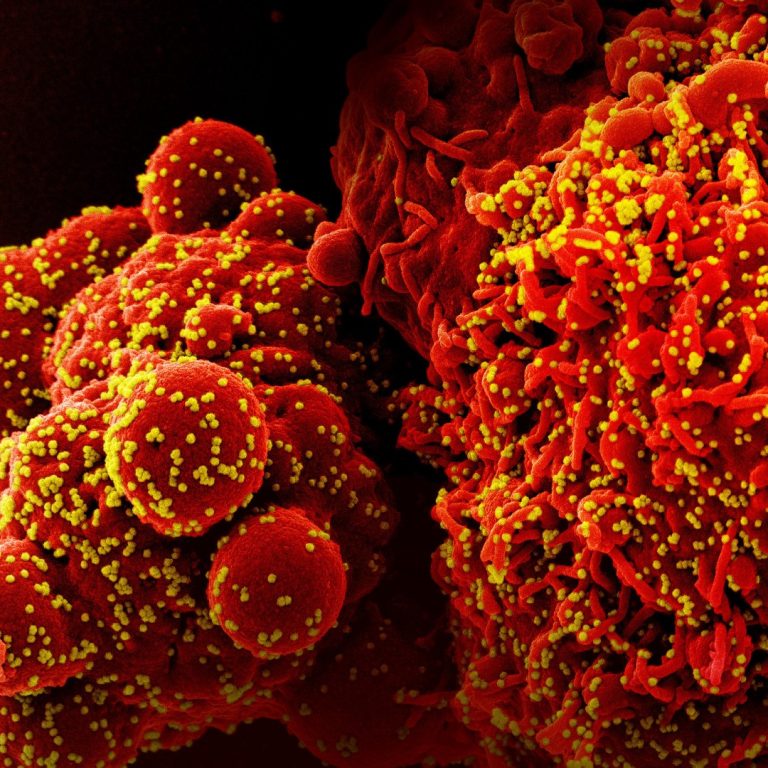
On Jun. 23, 2022, Innovation Pharmaceuticals reported that Brilacidin, the Company’s defensin-mimetic drug candidate exhibiting broad-spectrum antiviral activity,…

On Jun. 22, 2022, Merck announced that that the U.S. Food and Drug Administration (FDA) had approved an…

On Jun. 17, 2022, Moderna announced that it had received emergency use authorization from the U.S. Food and…

On Jun. 17, 2022, Pfizer and BioNTech announced the U.S. Food and Drug Administration (FDA) had granted emergency…

On Jun. 14 2022, Pfizer reported data from the Phase 2/3 EPIC-SR (Evaluation of Protease Inhibition for COVID-19…

On Jun. 13 2022, Novavax announced that the Australian Therapeutic Goods Administration (TGA) had granted provisional registration of…

On Jun. 10, 2022, the U.S. Department of Agriculture’s (USDA) National Veterinary Services Laboratory (NVSL) confirmed the presence…

On Jun. 8, 2022, Moderna announced new clinical data on its Omicron-containing bivalent COVID booster candidate, mRNA-1273.214, containing…

On Jun. 6, 2022, Pfizer further strengthened its commitment to United States manufacturing with a $120 million investment…

On Jun. 2, 2022, Moderna announced an agreement with the European Commission (EC) to amend their originally agreed…

On Jun. 2, 2022, the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service confirmed the presence…

On May 31, 2022, Moderna and Takeda announced the transfer of the marketing authorization for Moderna’s COVID-19 vaccine…

On May 31, 2022, Novavax announced the initiation of its Phase 3 strain change trial to determine if…
On May 30, 2022, The Centre for Health Protection (CHP) of the Department of Health received notification from…