U.S. FDA approved first Respiratory Syncytial Virus (RSV) vaccine
On May 3, 2023, the U.S. FDA approved GlaxoSmithKline’s Arexvy as the first respiratory syncytial virus (RSV) vaccine…
On May 3, 2023, the U.S. FDA approved GlaxoSmithKline’s Arexvy as the first respiratory syncytial virus (RSV) vaccine…

On May 2, 2023, the U.S. FDA announced that it was taking additional steps to support the use…

On Apr. 27, 2023, Pfizer announced that experimental respiratory syncytial virus (RSV) vaccine was 82% effective in preventing…

On Apr. 26, 2023, Vertex announced the Food and Drug Administration (FDA) had approved the expanded use of…
On Apr. 25, 2023, the U.S. FDA approved Qalsody (tofersen) to treat patients with amyotrophic lateral sclerosis (ALS)…

On Apr. 24, 2023, the U.S. Food and Drug Administration (FDA) authorized Status COVID-19 Antigen Rapid Test for…

On Apr. 17, 2023, the U.S. FDA approved Gamida Cell’s Omisirge (omidubicel-onlv), a substantially modified allogeneic (donor) cord…

On Apr. 14, 2023, Revance Therapeutics announced that the U.S. Food and Drug Administration (FDA) had approved the…

On Apr. 14, 2023, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization for the…

On Mar. 29, 2023, the U.S. Food and Drug Administration (FDA) announced approval of Narcan, 4 milligram naloxone…

On Mar. 10, 2023, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved ZAVZPRET (zavegepant),…

On Mar. 9, 2023, the U.S. Food and Drug Administration (FDA) announced that it had published updates to…
On Mar. 8, 2023, Amphastar Pharmaceuticals announced that the U.S. Food and Drug Administration (FDA) had granted approval…

On Mar. 8, 2023, QuidelOrtho announced that it had been granted a De Novo request from the U.S….

On Mar. 6, 2023, Merck announced that the U.S. Food and Drug Administration had approved the addition of…

On Mar. 6, 2023, Neuromod Devices announced that the US Food and Drug Administration (FDA) had granted De…

On Mar. 1, 2023, BioNTech and Pfizer announced that they had submitted an application to the U.S. Food…

On Feb. 13, 2023, Novavax announced a modification to its existing agreement with the U.S. Department of Health…

On Feb. 10, 2023, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for…
On Feb. 8, 2023, BD announced that it had received Emergency Use Authorization from the U.S. Food and…
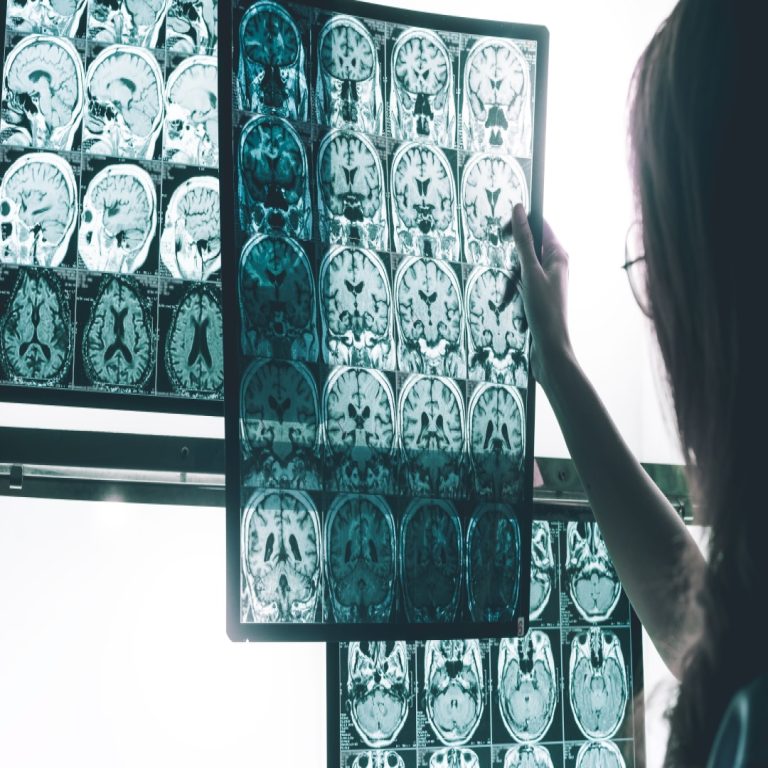
On Jan. 6, 2023, the U.S. Food and Drug Administration (FDA) approved Leqembi (lecanemab-irmb) via the Accelerated Approval…

On Dec. 24, 2022, the U.S. Food and Drug Administration (FDA) approved updated labeling for Genentech’s capecitabine tablets…

On Dec. 21, 2022, Roche announced that the U.S. Food and Drug Administration (FDA) had approved Actemraᆴ (tocilizumab)…

On Dec. 9, 2022, Pfizer and BioNTech announced the companies had received Fast Track Designation from the U.S….
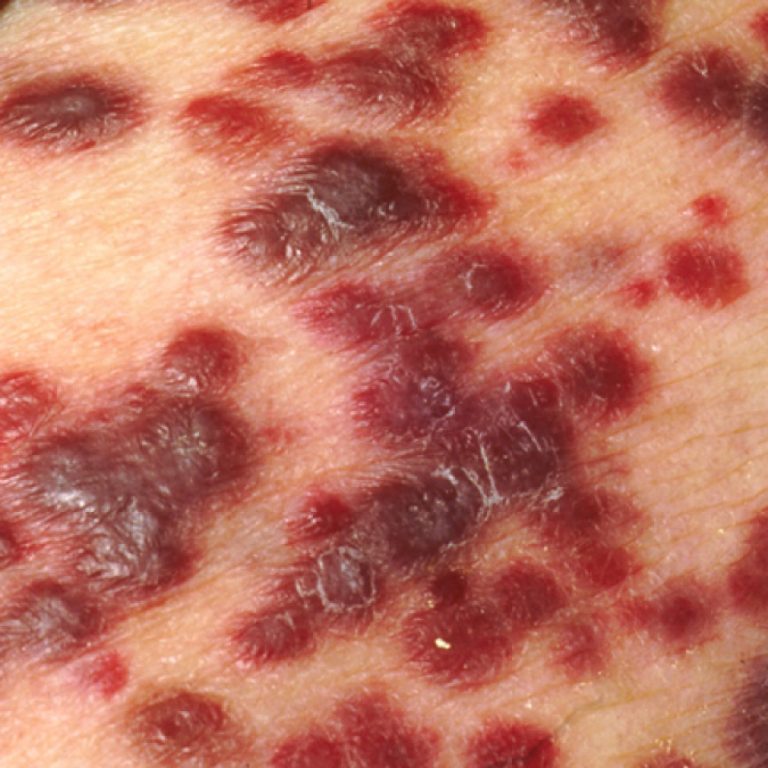
On Dec. 9, 2022, the U.S. Food and Drug Administration (FDA) approved Genentech’s atezolizumab (Tecentriq) for adult and…

On Dec. 8, 2022, Pfizer and BioNTech announced the U.S. Food and Drug Administration had granted Emergency Use…
On Dec. 7, 2022, Pfizer announced that the U.S. Food and Drug Administration (FDA) had accepted for priority…

On Nov. 17, 2022, the U.S. Food and Drug Administration (FDA) approved Tzield (teplizumab-mzwv) injection to delay the…

On Oct. 27, 2022, Inovio Pharma announced that it has discontinued its internally funded efforts to develop INO-4800…

On Oct. 19, 2022, the U.S. Food and Drug Administration final rule establishing Over-the-Counter Hearing Aids to improve…