The FDA approved the first test for HIV antbody screening of blood donors
On Mar. 2, 1985, the FDA announced the approval of the Abbott first antibody test kit to screen…

On Mar. 2, 1985, the FDA announced the approval of the Abbott first antibody test kit to screen…

On Sept. 24, 1984 the Hatch-Waxman Act (Drug Price Competition and Patent Term Restoration Act) was passed to…

On Dec. 18, 1982, Richard D. Palmiter at the University of Washington scientists in collaboration with Ralph L….

On Nov. 5, 1982, the U.S. Food and Drug Administration (FDA) published requirements of the tamper-resistant packaging (TRP)…

On Oct. 28, 1982, after only 5 months of review, the U.S. Food and Drug Administration (FDA) approved…

On Oct. 5, 1982, Johnson & Johnson, the parent company of McNeil, issued a nationwide recall of Tylenol…
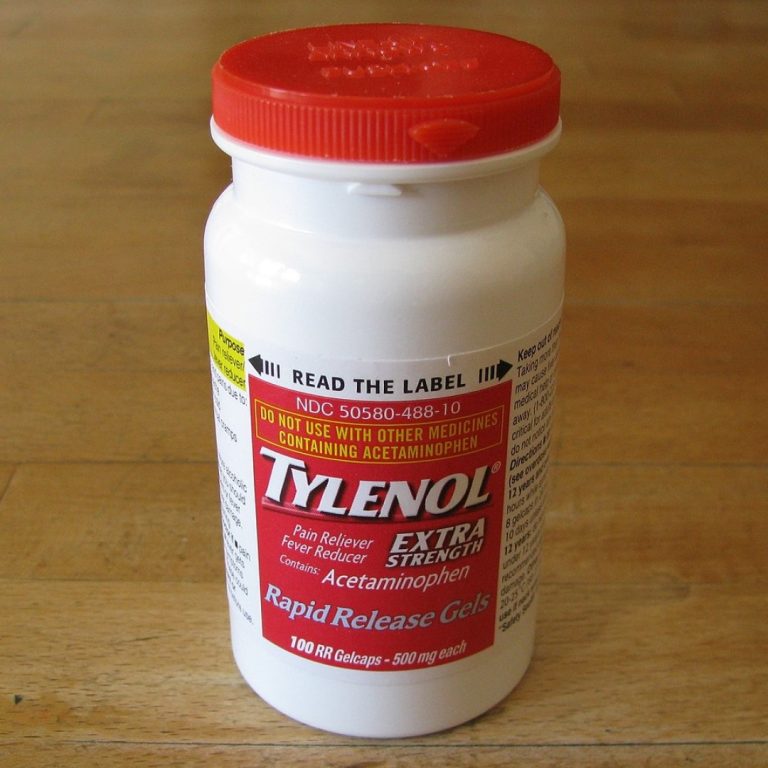
On Sept. 29, 1982. the first of seven victims died after taking a capsule of Extra-Strength Tylenol. On…
On Jan. 15, 1982, the second AIDS patient was admitted to the National Institute of Allergy and Infectious…

In 1982, the U.S. Food and Drug Administration issued a publication, known as the Redbook, that described the…
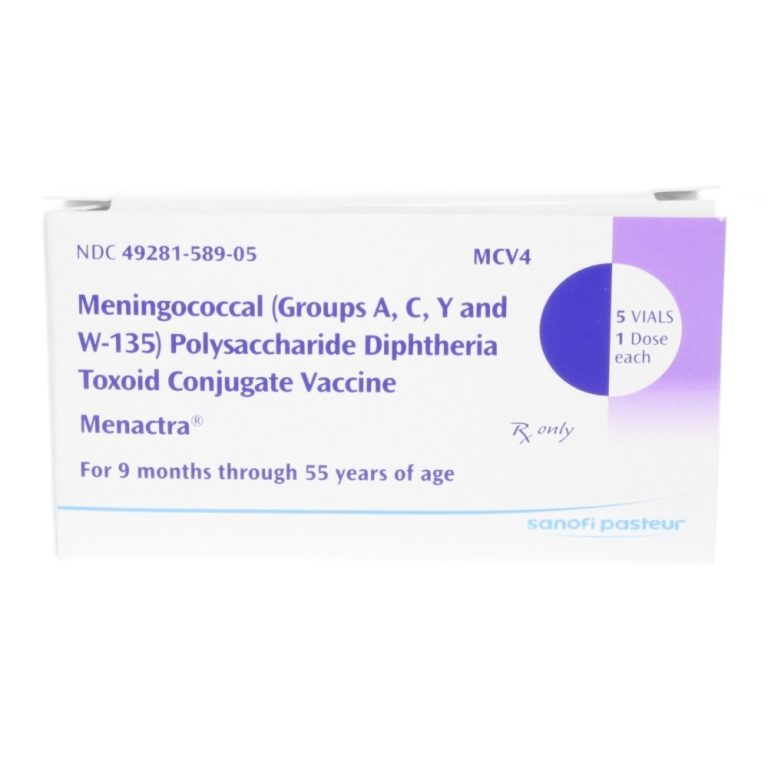
On Nov. 23, 1981, the U.S. Food and Drug Administration licensed Quadrivalent groups A, C, Y, and W-135…

On Jul. 2, 1981, the FDA approved vinblastine, a drug that binds to tubulin, the protein building block…

On Jan. 27, 1981, the U.S. Food and Drug Administration (FDA) and the U.S. Department of Health and…
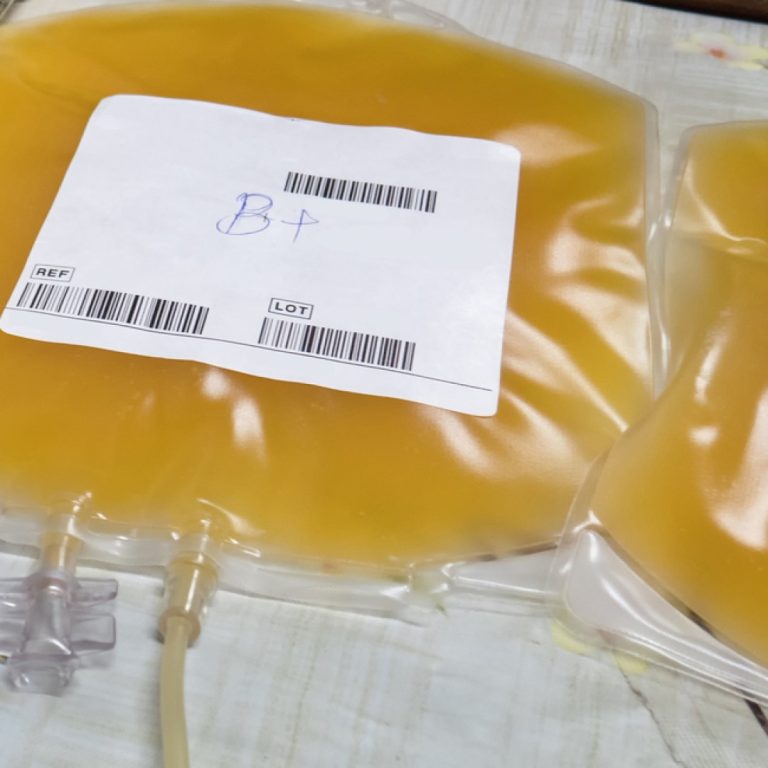
In 1981, the U.S. Food and Drug Administration (FDA) approved a plasma-derived hepatitis B vaccine for human use….

In 1981, Immunex Corporation was founded by Stephen Duzan, and Christopher Henney and Steven Gillis from the Hutchinson…

In 1981, Imre Corp. (Immune Response Systems, Inc.) was founded in Seattle. The company developed the Prosorba Column,…

In 1980, the Infant Formula Act is one of the most specific and detailed acts ever passed by…
On Dec. 19, 1978, the U.S. Food and Drug Administration (FDA) approved cisplatin (Platinol) for use in combination…
On Jan. 3, 1978, the Yellow fever vaccine (YF-Vax by Connaught) was licensed in the U.S. The Yellow…
On Dec. 30, 1977, the U.S. Food and Drug Administration approved Tamoxifen, an anti-estrogen drug, for the treatment…

On Nov. 23, 1977, the Saccharin Study and Labeling Act was enacted by the U.S. Congress to stop…
On Nov. 21, 1977, the U.S. Food and Drug Administration (FDA) licensed the first pneumococcal vaccine containing 14…

On Apr. 4, 1977, Donald Kennedy, Ph.D., became Commissioner of the U.S. Food and Drug Administration (FDA). Kennedy,…

In 1977, the U.S. Food and Drug Administration (FDA) established the Bioresearch Monitoring Program (BiMo) to develop cross-center…

On Apr. 22, 1976, the U.S. Congress passed the Vitamin-Mineral Amendment to the Federal Food, Drug, and Cosmetic…

In 1976, Influenza A/Victoria-like strains had been identified in New Jersey as early as January 21. The novel…

In 1976, the U.S. Congress passed the Medical Device Amendments to ensure safety and effectiveness of medical devices,…

In 1975, rapamycin, a macrolide produced by the bacterium Streptomyces hygroscopicus was first discovered and isolated from soil…

In Jul. 1974, In the Division of Pharmaceutical Service at the University of Iowa began producing cGMP compliant…

In 1974, the U.S. Food and Drug Administration (FDA) approved doxorubicin (Adriamycin), an antitumor anthracycline antibiotic from Streptomyces…

On Jun. 18, 1973, the U.S. Supreme Court upheld the 1962 drug effectiveness law and endorsed Food and…