The FDA approved PRP-OMP for routine administration to infants beginning at 2 months of age
On Dec. 13, 1990, the U.S. Food and Drug Administration (FDA) approved Haemophilus b Conjugate Vaccine (Meningococcal Protein…
On Dec. 13, 1990, the U.S. Food and Drug Administration (FDA) approved Haemophilus b Conjugate Vaccine (Meningococcal Protein…
On Nov. 28, 1990, the Safe Medical Devices Act was passed, requiring nursing homes, hospitals, and other facilities…
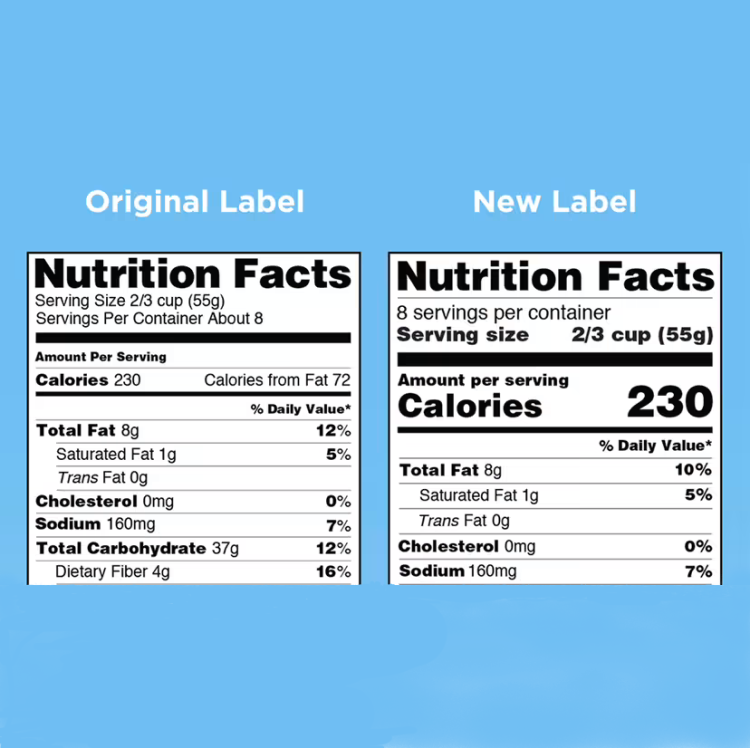
On Nov. 8, 1990, the Nutrition Labeling and Education Act (NLEA) was signed into law by President George…

On Dec. 29, 1989, the U.S. Food and Drug Administration (FDA) approved Oculinum’s (onabotulinumtoxinA) for treatment of strabismus…

On Nov. 24, 1989, the U.S. Food and Drug Administration (FDA) issued a nationwide recall of all over-the-counter…
On Aug. 28, 1989, a second recombinant hepatitis B vaccine, Engerix-B (SmithKlineBeecham), was approved for use in the…

On Jun. 1, 1989, the FDA approved Amgen’s Epogen/Procrit for the treatment of anemia associated with chronic renal…

On Mar. 3, 1989, carboplatin (Paraplatin), a drug related to cisplatin, was approved by the U.S. Food and…

On Mar. 3, 1989, the U.S. Food and Drug Administration (FDA) approved Bristol-Myers’ PARAPLATIN (carboplatin) for the treatment…

In 1989, transgenic fast-growing salmon were created. In 2015, the U.S. Food and Drug Administration (FDA) approved a…

In 1989, CellPro was founded in Bothell as a spin-off from the Fred Hutchinson Cancer Research Center. The…

On Dec. 21, 1988, the conjugated Haemophilus influenzae type b vaccine (HibTITER by Wyeth-Lederle) was licensed. Prior to…

On Apr. 22. 1988, the Prescription Drug Marketing Act of 1987 (PDMA) was signed into law by the…

In 1988, the Food and Drug Administration Act by the U.S. Congress officially established the U.S. Food and…

In 1988, the U.S. Food and Drug Administration (FDA) published a final rule, Annual Summary Reporting Requirements Under the…
In 1988, Dr. Fletcher Taylor and Dr. Charles Esmon from the Oklahoma Medical Research Foundation (OMRF) filed for…
On Dec. 22, 1987, the protein-conjugated Haemophilus influenzae type b vaccine (PRP-D, ProHibit by Connaught) was licensed.

In December 1987, the antidepressant fluoxetine (Prozac), discovered by David T. Wong, received U.S. Food and Drug Administration…

On Oct. 6, 1987, the Center for Drugs and Biologics within the U.S. Food and Drug Administration was…

On Jun. 8, 1987, Advanced Genetic Sciences announced that its Frostban (Ice-minus) bacteria successfully protected strawberries from below-freezing…

In 1987, the U.S. Food and Drug Administration (FDA) announced revised regulations regarding Expanded Access (EA) to investigational…
In 1987, the FDA approved first Western blot blood test more specific HIV diagnostic test and AZT as…

In 1987, the U.S. Food and Drug Administration (FDA) approved Genentech’s drug tPA Activase (alteplase). Activase is a…

On Oct. 27, 1986, President Ronald Reagan signed the the Anti-Drug Abuse Act of 1986. The Act changed…

On Aug. 6, 1986, Bill Schroeder from Jasper, Indiana, the second human recipient of the Jarvik 7 artificial…
On Jun. 5, 1986, the U.S. Food and Drug Administration (FDA) approved Hoffmann La Roche’s Interferon initially for…
In May 1986, the vaccine Recombivax HB, which protects against hepatitis B infection, was approved for marketing in…

In 1986, Procyte Corp. was founded as a Kirkland, Washington-based medical skin care company that developed and marketed…

In Dec. 1985, the first clinical tests were held at the University of Washington of erythropoietin (EPO), the…

On Nov. 20, 1985, the Health Research Extension Act of 1985 (P.L. 99-158) was signed into law by…