Rimantadine was approved by the FDA to treat influenza A
In 1994, Rimantadine, derived from amantadine, was approved by the Food and Drug Administration (FDA) to treat influenza…

In 1994, Rimantadine, derived from amantadine, was approved by the Food and Drug Administration (FDA) to treat influenza…

In 1994, Omeros Corp. was founded in Seattle as a biopharmaceutical company committed to discovering, developing and commercializing…

In 1994, Bristol-Myers Squibb’s PRAVACHOL (pravastatin sodium) was granted expanded usage from the U.S. Food and Drug Administration…

On Dec. 30, 1993, the Genentech drug Pulmozyme (dornase alfa) was approved by the U.S. Food and Drug…
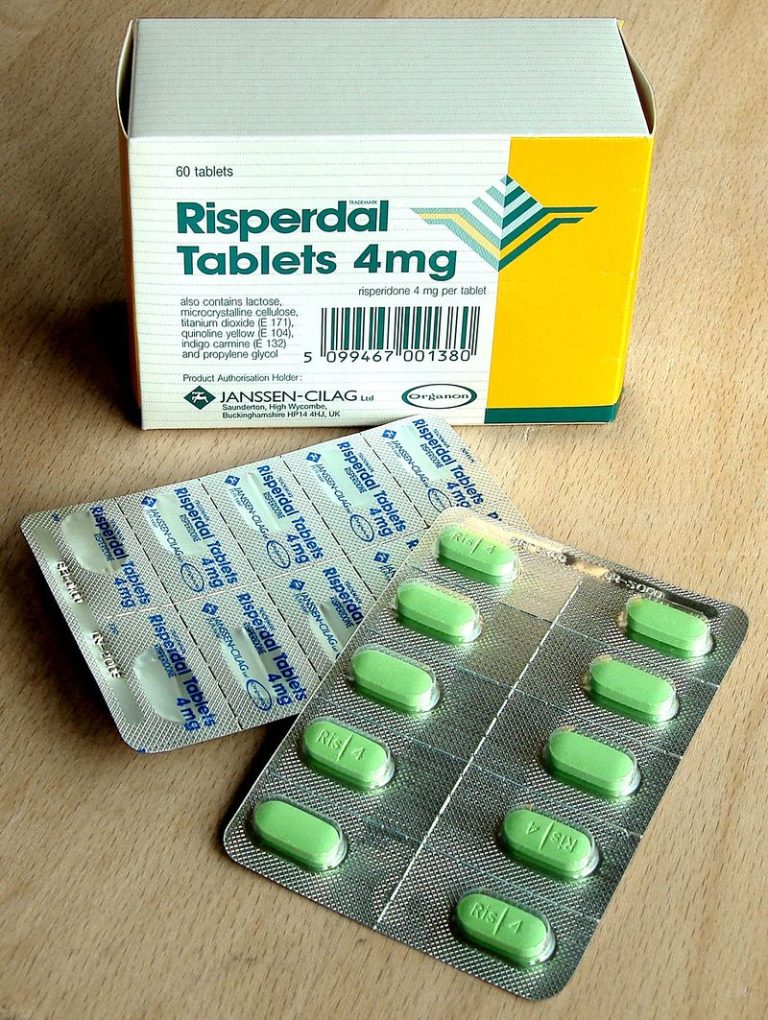
On Dec. 29, 1993, the U.S. Food and Drug Administration (FDA) approved indications for oral risperidone (tablets, oral…

On Nov. 17, 1993, Genentech drug Nutropin was approved by the U.S. Food and Drug Administration (FDA) for…

On Nov. 12 ,1993, FDA granted approval for the sale of the Ahmed Glaucoma Valve designed and manufactured…

On Jul. 23, 1993, the U.S. Food and Drug Administration (FDA) approved Bayer’s Betaseron, the first of several…
On Jul. 22, 1993, revising a policy from 1977 that excluded women of childbearing potential from early drug…

On Apr. 14, 1993, the Earl Davie/ZymoGenetics Endowed Chair of Biochemistry at the University of Washington was established…
On Apr. 1, 1993, the FDA approved Amgen’s Epogen/Procrit for the treatment of anemia due to the effects…

On Mar. 30, 1993, the U.S. Food and Drug Administration (FDA) approved a new Haemophilus b conjugate vaccine,…

In 1993, the U.S. Food and Drug Administration (FDA) declared genetically-engineered foods are not inherently dangerous and do…

In 1993, The U.S. Food and Drug Administration (FDA) added new CBER reviewers to streamline biologics review. User…

On Dec. 18, 1992, Taxol (paclitaxel), an anticancer drug extracted from the bark of the Pacific yew, received…
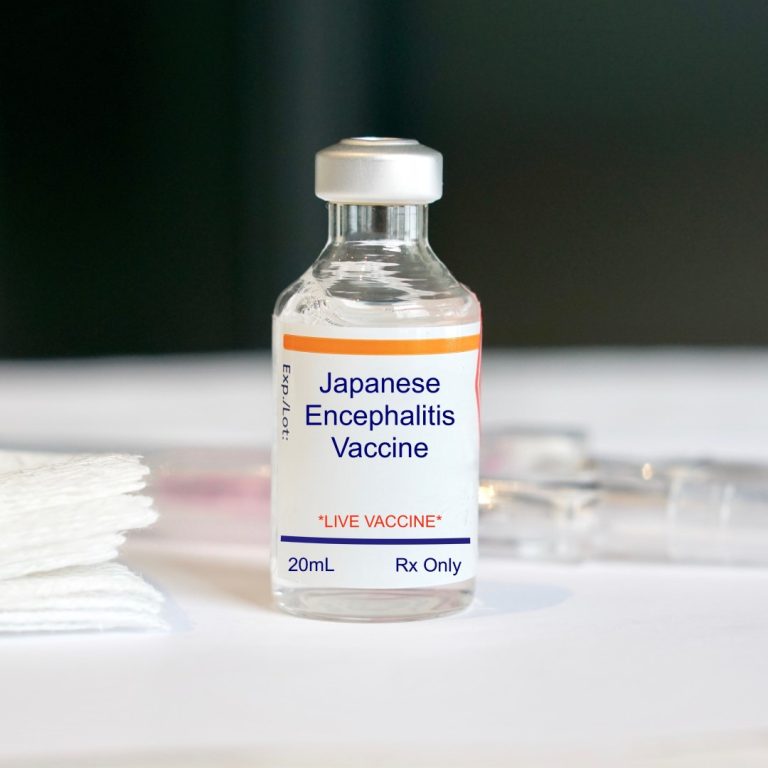
On Dec. 10, 1992, the Japanese encephalitis (JE) virus vaccine inactivated (JE-Vax by Research Foundation for Microbial Diseases…
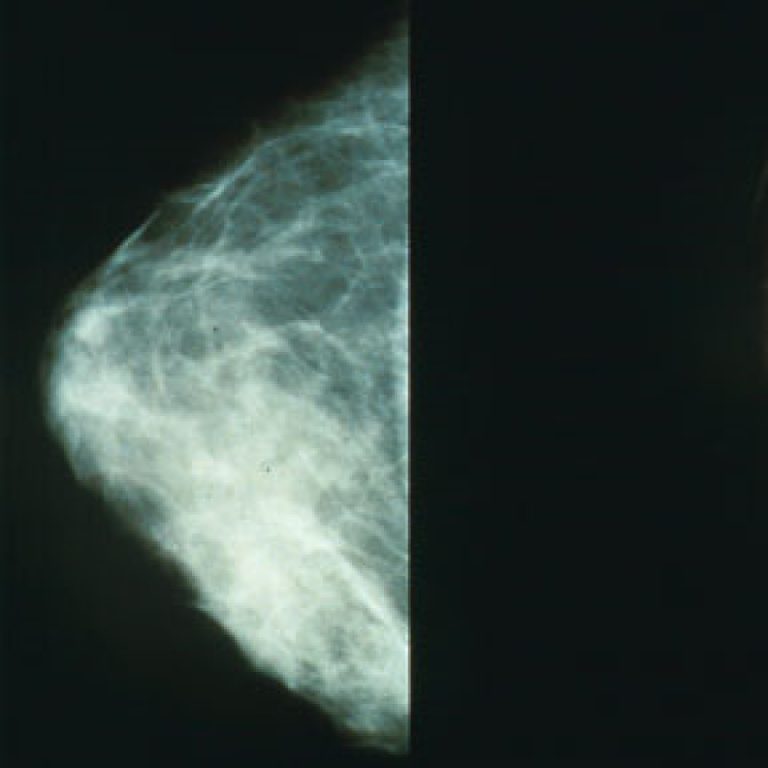
On Oct. 27, 1992, the Mammography Quality Standards Act (MQSA) was passed (P.L. 102-539) by the U.S. Congress…

On Sept. 3, 1992, PathoGenesis Corp. was founded by Robert Nowinski. The company, based in Seattle, developed an…

On Feb. 29, 1992, the U.S. Food and Drug Administration (FDA) announced new food labeling rules. The purpose…

In 1992, President George Bush signed the Prescription Drug User Fee Act (PDUFA) and under the Clinton administration,…

In 1992, the University of Iowa (UI) Research Foundation received its first U.S. patent on the CMV promoter,…
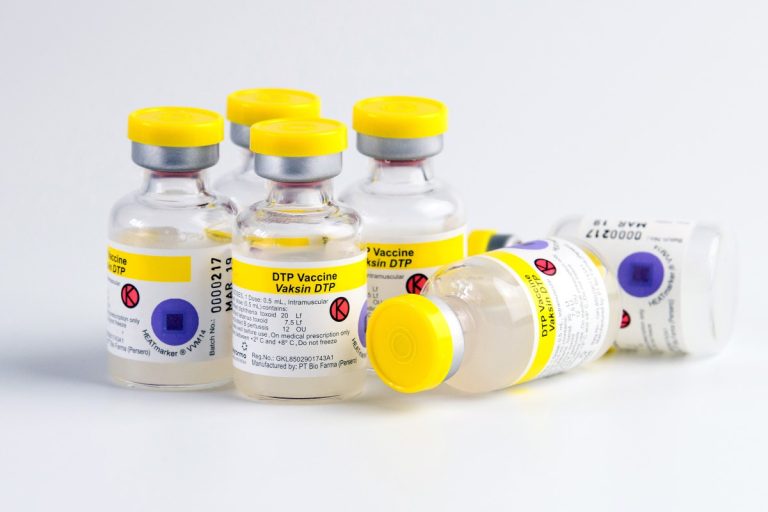
On Dec. 17, 1991, the Diphtheria and tetanus toxoids and acellular pertussis vaccine (Acel-Imune by Lederle) was licensed…

On Oct. 9, 1991, Bristol-Myers’ VIDEX (didanosine) was approved by the U.S. Food and Drug Administration for the…

On Aug. 26, 1991, Cellpro announced its initial public offering. CellPro, founded in Bothell, Washington, developed the CEPRATE…

On Jun. 6, 1991, ICOS completed its IPO at at $8.00 per share for a total of $36…
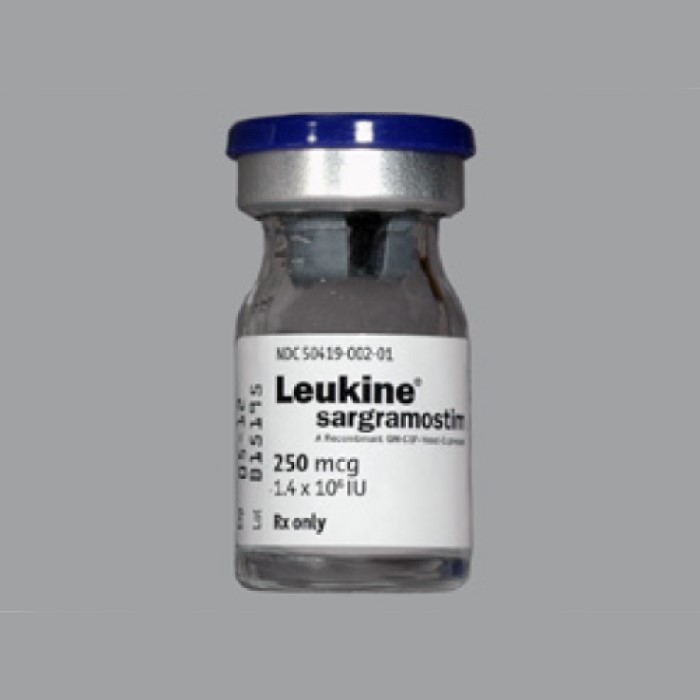
In March 1991, Immunex announced it had received U.S. Food and Drug Administration (FDA) approval to market Leukine…
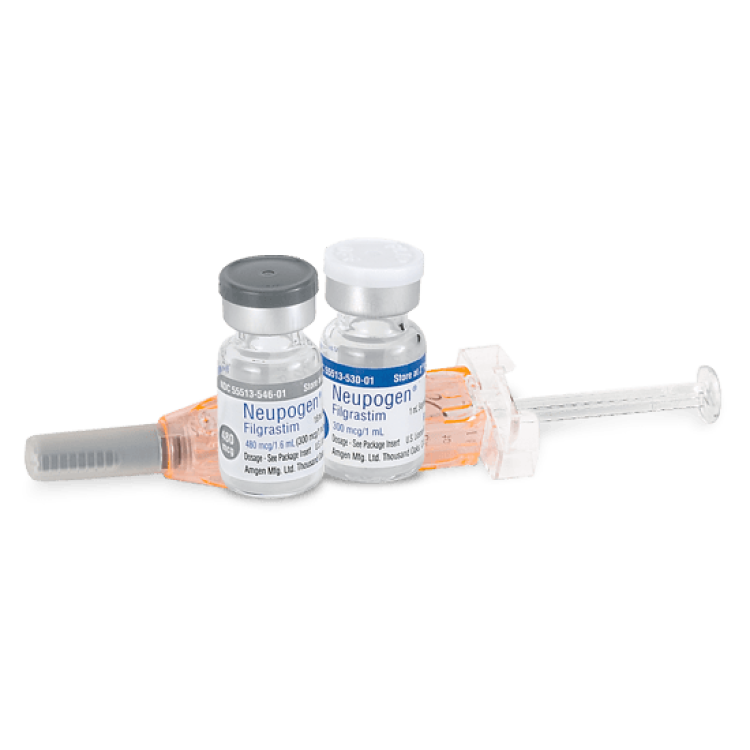
On Feb. 22, 1991, the Food and Drug Administration (FDA) announced that it had approved Amgen’s two white…

In 1991, the policy for protection of human subjects in research, promulgated in 1981 by the U.S. Food…

In 1991, the U.S. Food and Drug Administration (FDA) published regulations to Accelerate the Review of Drugs for life-threatening diseases….

In 1991, Bristol Myers Squibb’s cardiovascular medicines, PRAVACHOL (pravastin sodium) and MONOPRIL (fosinopril sodium) were approved by the…