Monoclonal antibody rituximab (Rituxan) approved by the FDA to treat patients with non-Hodgkin lymphoma
On Nov. 26, 1997, the U.S. Food and Drug Administration (FDA) announced it had approved the Monoclonal antibody…
On Nov. 26, 1997, the U.S. Food and Drug Administration (FDA) announced it had approved the Monoclonal antibody…

On Nov. 21, 1997, the FDA Modernization Act (FDAMA) was signed into law, amending the Food, Drug and…
On Oct. 20, 1997, the Food and Drug Administration (FDA) licensed Purified Chick Embryo Cell (PCEC, RabAvert®) vaccine…

On Aug. 9, 1997, the U.S. Food and Drug Administration (FDA) announced a policy that allowed pharmaceutical companies…

On Dec. 30, 1996, the Food and Drug Administration (FDA) announced it had licensed three DTaP vaccines for…

On Oct. 9, 1996, the Animal Drug Availability Act added flexibility to animal drug approval process, providing for…
On Jul. 31, 1996, the diphtheria and tetanus toxoids and acellular pertussis vaccine adsorbed (Tripedia by Aventis Pasteur)…

On Jul. 1, 1996, topotecan (Hycamptin), the first of a class of drugs that interferes with the enzyme…

On Apr. 9, 1996, the Federal Tea Tasters Repeal Act of 1996 eliminated the Board of Tea Experts…

On Mar. 14, 1996, the U.S. Food and Drug Administration (FDA) announced the approval of the first antigen…

In 1996, the first genetically engineered crop was commercialized. The first genetically modified plant was produced a short…
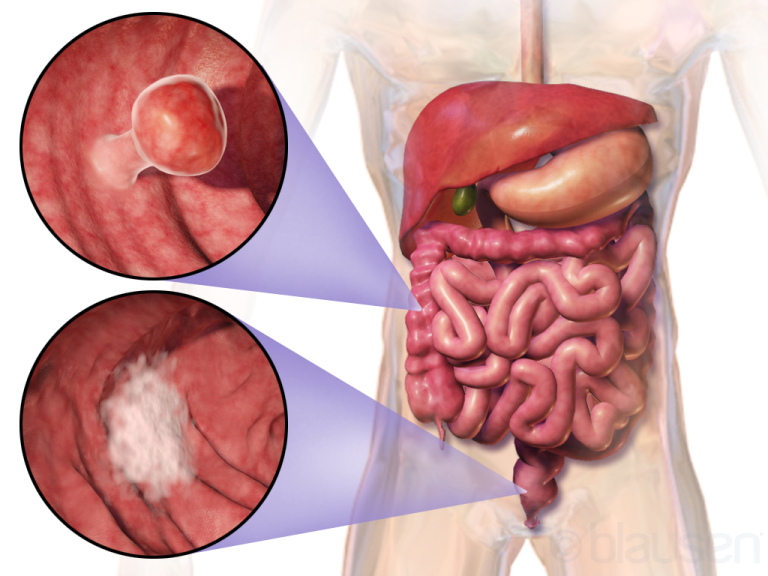
In June 1996, the Food and Drug Administration (FDA) first approved irinotecan for the treatment of patients with…

On Dec. 11, 1996, scientists from Stanford University and Affymetrix announced a new high-tech method that used a…

On Dec. 29, 1995, the Genentech drug Nutropin AQ ((somatropin) injection for subcutaneous use) was approved by the…
Dec. 27, 1995, the U.S. Food and Drug Administration (FDA) approved anastrozole (Arimidex) as a treatment for breast…

On Dec. 9, 1995, the U.S. Food and Drug Administration (FDA) approved tretinoin, a differentiating agent related to…

On Dec. 6, 1995, the U.S. Congress repealed the saccharin notice requirements. The store warning notice requirement was…

In 1995, Porfimer sodium, a light-sensitive drug that can be absorbed by tumors, was approved by the U.S. Food…

On Sept. 27, 1995, a series of proposed reforms to reduce regulatory burden on pharmaceutical manufacturers were announced….

On May 3, 1995, the U.S. Food & Drug Administration (FDA) announced it had approved Genentech’s drug CellCept…

In 1995, the FDA declared cigarettes to be “drug delivery devices.” Restrictions were proposed on marketing and sales…

In 1995, the Immunization Practices Advisory Committee (ACIP), American Academy of Pediatrics, and the American Association of Family…
On Dec. 23, 1994, the FDA announced the approval of the first U.S. HIV test system using oral…

On Oct. 25. 1994, the Dietary Supplement Health and Education Act was enacted establishing specific labeling requirements, provided…

On Oct. 22, 1994, the Animal Medicinal Drug Use Clarification Act (AMDUCA) became law permitting veterinarians to prescribe…

On May 17, 1994, the U.S. Food and Drug Administration (FDA) approved of the first genetically modified food:…
On Feb. 25, 1994, the U.S. Food and Drug Administration (FDA) announced it could consider regulating regulating tobacco…

In 1994, Rimantadine, derived from amantadine, was approved by the Food and Drug Administration (FDA) to treat influenza…

In 1994, Omeros Corp. was founded in Seattle as a biopharmaceutical company committed to discovering, developing and commercializing…

In 1994, Bristol-Myers Squibb’s PRAVACHOL (pravastatin sodium) was granted expanded usage from the U.S. Food and Drug Administration…