Zostavax was approved by the FDA to prevent shingles (herpes zoster) in individuals 60 years of age and older
On May 25, 2006, Zostavax, manufactured by Merck & Co. of Whitehouse Station, New Jersey, was approved by…

On May 25, 2006, Zostavax, manufactured by Merck & Co. of Whitehouse Station, New Jersey, was approved by…

On Feb. 24, 2006, the Advisory Committee on Immunization Practices (ACIP) was informed by the only U.S.-licensed manufacturer…
In 2006, Calistoga Pharmaceuticals was founded as a Seattle-based spin-off from ICOS Corp. that was dedicated to developing…
On Dec. 19, 2005, in a 2-year legal battle over the U.S. military’s anthrax vaccination program, the Food…

On Dec. 12, 2005, the U.S. Congress approved the passage of the Food Allergy Labeling and Consumer Protection…
On Oct. 17, 2005, the FDA approved lowering the age limit to 12 mos for the remaining U.S.-licensed…
On Aug. 11, 2005, the Food and Drug Administration (FDA) approved an application of a pediatric/adolescent formulation of…
On Jun. 9, 2005, the U.S. Food and Drug Administration (FDA) licensed a 2nd Tdap vaccine (Adacel by…

On Apr. 29, 2005, Amylin Pharmaceuticals and Eli Lilly announced the FDA had approved BYETTA(TM) (exenatide) injection as…
On Apr. 22, 2005, Sanofi Pasteur announced the U.S. Food and Drug Administration (FDA) had granted licensure to…

On Feb. 16, 2005, in preparation for the new generation of molecular-based oncology medical products, the National Cancer…

On Feb. 16, 2005, Formation of the Drug Safety Board was announced, consisting of FDA staff and representatives…

On Feb. 15, 2005, in response to concerns about the safety of a number of prescription medicines, the…

In Jan. 2005, the U.S. Food and Drug Administration (FDA) approved an albumin-stabilized nanoparticle formulation of paclitaxel (Abraxane)…

On Dec. 15, 2004, Amgen announced that following priority review, the FDA has approved Kepivance(TM) (palifermin), the first…

On Nov. 18, 2004, the U.S. Food and Drug Administration (FDA) announced it had approved Genentech’s drug Tarceva…
On Oct. 29, 2004, the U.S. Food and Drug Administration (FDA) approved Letrozole for the extended adjuvant treatment…

On Sept. 30, 2003, University of Iowa (UI) Microbiology Professor Mark Stinski made the discoveries of CMV promoter…

On Aug. 25, 2004, a significant shortage of influenza vaccine occurred in the U.S. as a result of…

On Aug. 24, 2004, Eli Lilly and Company launched Cymbalta (duloxetine HC), a new treatment for major depression…

On Jul. 21, 2004, Project BioShield Act of 2004 was signed by President George W. Bush. The Act…

On Apr. 19, 2004, Eli Lilly and Company launched Symbyax in the U.S., the first and only Food…

On Mar. 24, 2004, the U.S. Food and Drug Administration (FDA) licensed tetanus and diphtheria toxoids adsorbed for…
On Mar. 16, 2004, the Food and Drug Administration (FDA) released a report addressing the recent slowdown in…
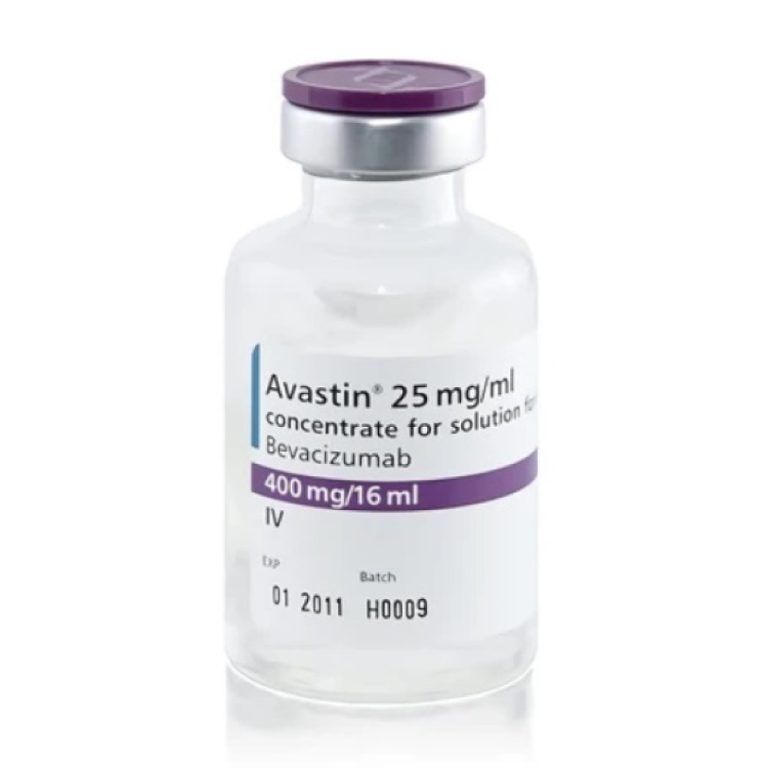
On Feb. 26, 2004, the U.S. Food and Drug Administration (FDA) announced it had approved the first antiangiogenic…

On Feb. 9, 2004, deeming such products to present an unreasonable risk of harm, the FDA banned dietary…
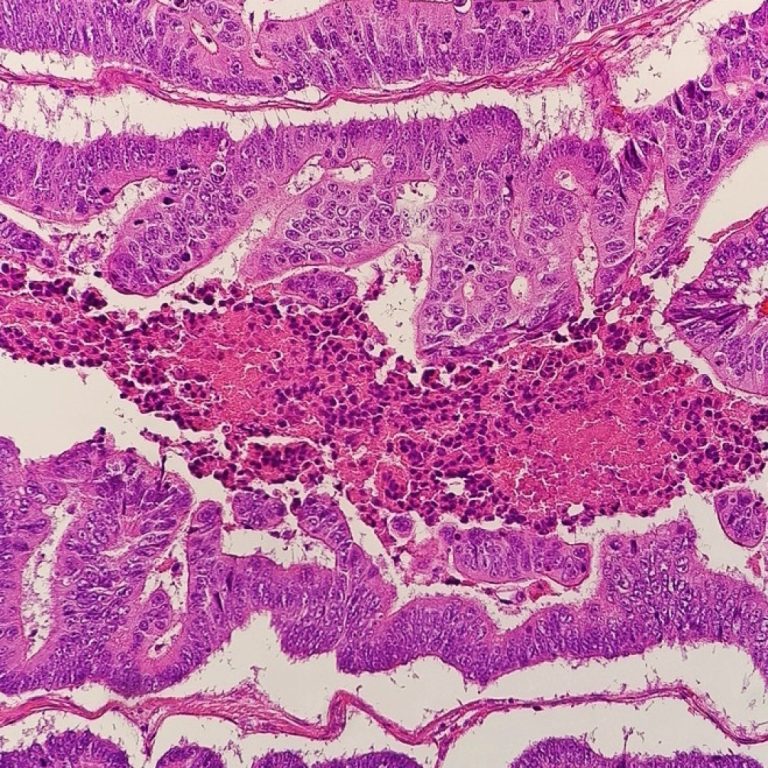
On Feb. 1, 2004, the U.S. Food and Drug Administration (FDA) announced it had approved Cetuximab, a recombinant…
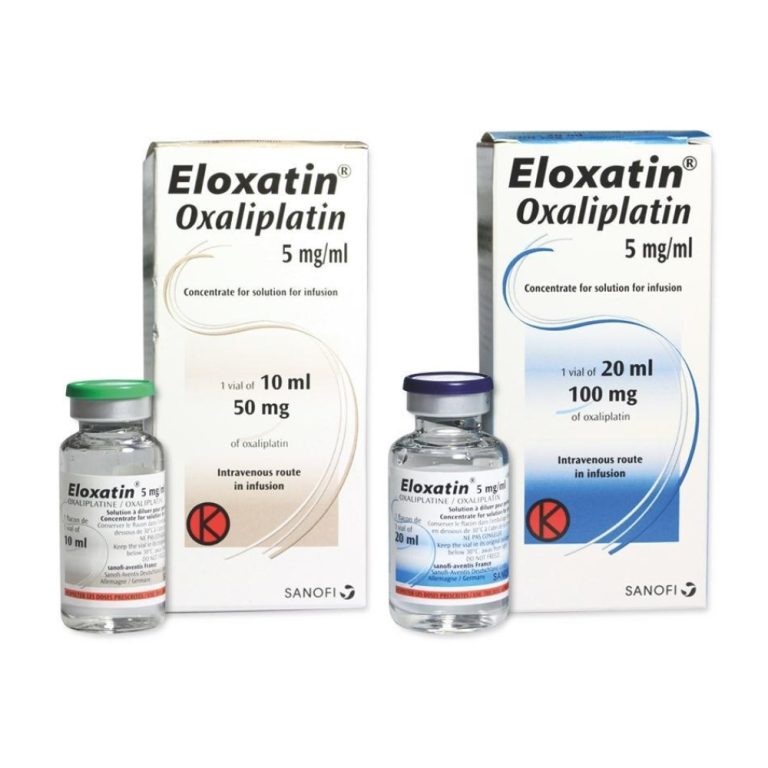
On Jan. 9, 2004, the Food and Drug Administration (FDA) announced the approval of Sanofi’s Eloxatin (oxaliplatin) injection…
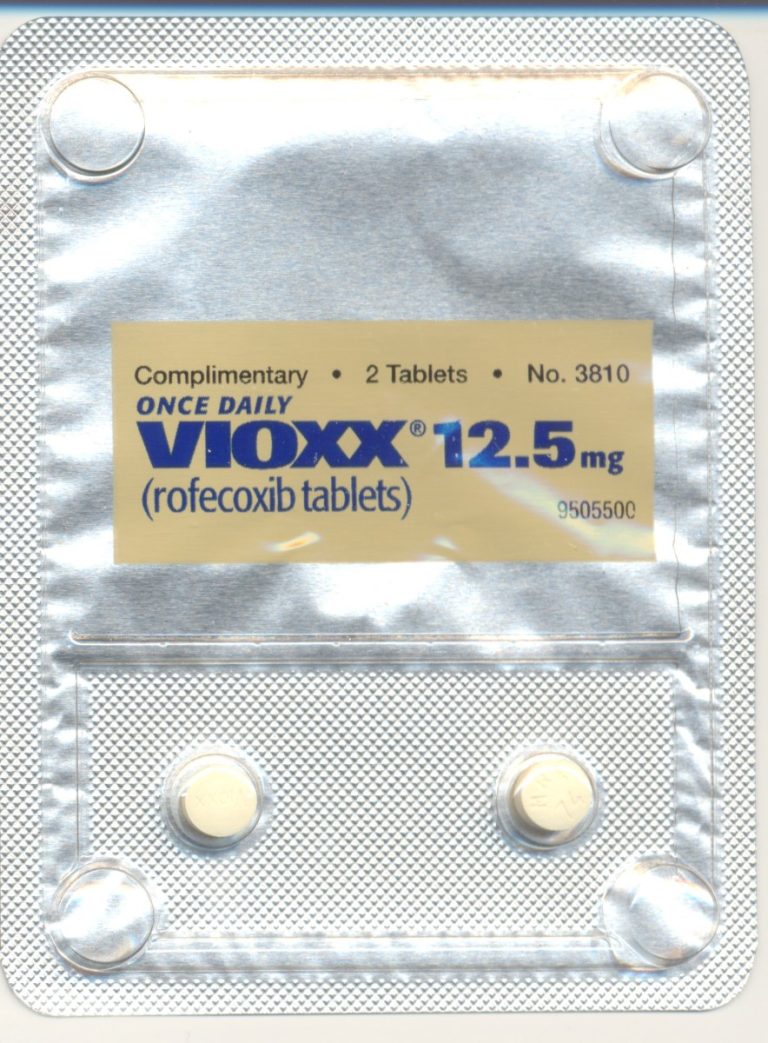
In 2004, Merck announced a voluntary worldwide withdrawal of Vioxx (rofecoxib) because of an increased risk of heart…

In 2004, the FDA published “Innovation or Stagnation? — Challenge and Opportunity on the Critical Path to New…