FDA approved Cervarix, GlaxoSmithKline’s cervical cancer vaccine
On Oct. 16, 2009, GlaxoSmithKline announced the U.S. Food and Drug Administration (FDA) had approved Cervarix [Human papillomavirus…

On Oct. 16, 2009, GlaxoSmithKline announced the U.S. Food and Drug Administration (FDA) had approved Cervarix [Human papillomavirus…
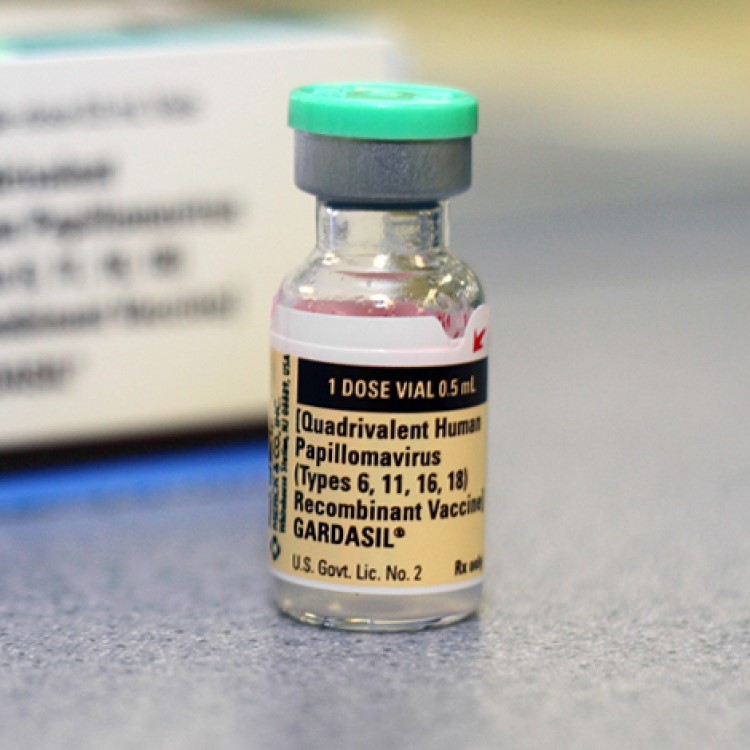
On Oct. 16, 2009, the U.S. Food and Drug Administration (FDA) approved new indication for gardasil to prevent…
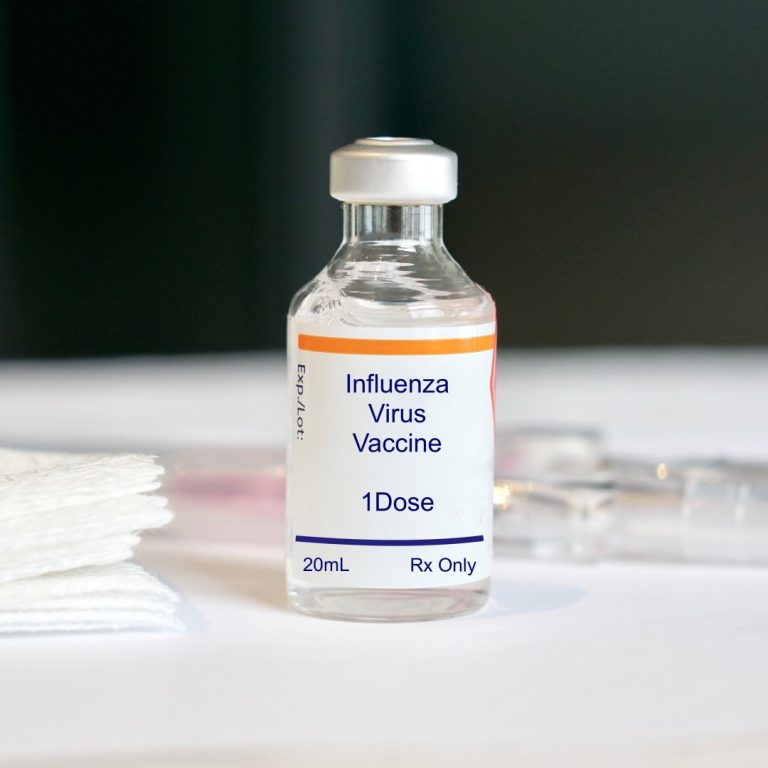
On Sept. 15, 2009, the U.S. Food and Drug Administration (FDA) approved four Influenza A (H1N1) 2009 Monovalent…
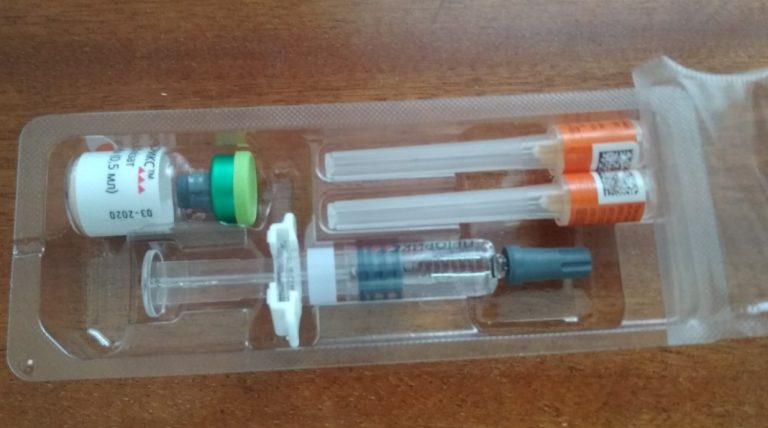
On Feb. 12, 2009, the Vaccine Court ruled that MMR vaccine, when administered with thimerosal-containing vaccines, does not…

On Feb. 6, 2009, the U.S. Food and Drug Administration (FDA) approved the first genetically engineered animal for…
On Dec. 19, 2008, the Food and Drug Administration (FDA) approved changes in the schedule for administering anthrax…
On Dec. 4, 2008, the Food and Drug Administration (FDA) approved an expanded age indication for the tetanus…

On Nov. 19, 2008, the U.S. Food and Drug Administration opened its first foreign office in Beijing to…

On Jun. 24, 2008, the U.S. Food and Drug Administration (FDA) licensed a combined diphtheria and tetanus toxoid…

On Jun. 23, 2008, Sanofi Pasteur announced the U.S. Food and Drug Administration (FDA) had licensed Pentacel, Diphtheria…
On Jun. 20, 2008, the FDA approved Pentacel (Sanofi Pasteur), a new combination DTaP-IPV-Hib vaccine for use in…

On Jun. 5, 2008, the FDA approved the use of Sanofi Pasteur’s Tenivac tetanus and diphtheria toxoids adsorbed…
On Apr. 3, 2008, the U.S. Food and Drug Administration (FDA) approved new rotavirus vaccine (Rotarix) for use…

On Jan. 17, 2008, ZymoGenetics received U.S. Food and Drug Administration (FDA) authorization to manufacture the product Thrombin…

On Jan. 8, 2008, Eli Lilly announced that the U.S. Food and Drug Administration (FDA) had approved Cialis(R)…

On Sept. 28, 2007, the U.S. Food and Drug Administration (FDA) approved an Australian-made influenza vaccine called Afluria…

On Sept. 27, 2007, the FDA Amendments Act of 2007 amended the Federal Food, Drug, and Cosmetic Act…

On Sept. 20, 2007, the U.S. Food and Drug Administration (FDA) approved use of FluMist nasal-spray influenza vaccine…

On Sept. 14, 2007, the U.S. Food and Drug Administration (FDA) announced it had approved Eli Lilly’s osteoporosis…

On Aug. 16, 2007, surgeons at Oregon Health and Science University announced implanting a new artificial disc in…

On Apr. 17, 2007, Sanofi announced that the U.S. Food and Drug Administration (FDA) had licensed its H5N1…
On Mar. 28, 2007, the U.S. Food and Drug Administration (FDA) announced that GlaxoSmithKline (King of Prussia, Pennsylvania)…

On Jan. 29, 2007, Eli Lilly announced it had completed its acqusition of ICOS Corp. for $2.3 billion…

On Jan. 9, 2007, the U.S. Food and Drug Administration (FDA) announced it had approved a refrigerated form…

In 2007, the U.S. Food and Drug Administration (FDA) approved Elelyso (taliglucerase alfa) for long-term enzyme replacement therapy…
On Nov. 16, 2006, the U.S. Food and Drug Administration (FDA) approved Genentech’s trastuzumab (Herceptin) for use with…
On Oct. 19, 2006, the U.S. Food and Drug Administration (FDA) granted Glivec® (imatinib) has received US regulatory…

On Oct. 19, 2006, the U.S. Food and Drug Administration (FDA) approved the drug, Gleevec developed by Oregon…

On Oct. 18, 2006, ICOS announced it had signed an agreement to be acquired by Eli Lilly and…

On Oct. 5, 2006, the Foundation for the National Institutes of Health, NIH, FDA, and the Pharmaceutical Research…