FDA expanded age indication for Menveo to include infants and toddlers age 2 through 23 months
On Aug. 1, 2013, Novartis announced the U.S. Food and Drug Administration (FDA) had approved Menveo (Meningococcal [Groups…
On Aug. 1, 2013, Novartis announced the U.S. Food and Drug Administration (FDA) had approved Menveo (Meningococcal [Groups…
On Jun. 10, 2013, Sanofi Pasteur announced the U.S. Food and Drug Administration (FDA) had approved the supplemental…

On Feb. 22, 2013, the U.S. Food and Drug Administration (FDA) announced they had approved Genentech’s licensed ado-trastuzumab…

On Jan. 25, 2013, Pfizer announced the FDA had granted approval for the expansion of the companyメs pneumococcal…
On Dec. 14, 2012, the U.S. Food and Drug Administration (FDA) announced it had approved GlaxoSmithKline’s quadrivalent formulation…
On Nov. 20, 2012, the U.S. Food and Drug Administration (FDA) announced it had approved first seasonal influenza…
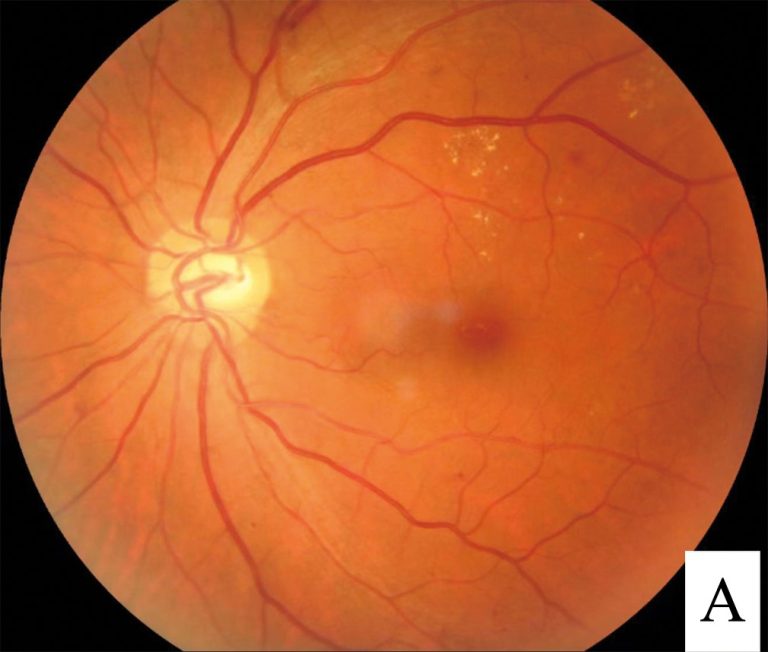
On Aug. 10. 2012, Genentech announced that Lucentis (ranibizumab injection) was approved by the U.S. Food and Drug…

On Jul. 9, 2012, the FDA created the “Breakthrough Therapy Designation” that expedites development and review of drugs…

On Jun. 24, 2012, the U.S. Food and Drug Administration (FDA) announced it had approved HibMenCY (Menhibrix, GlaxoSmithKline),…
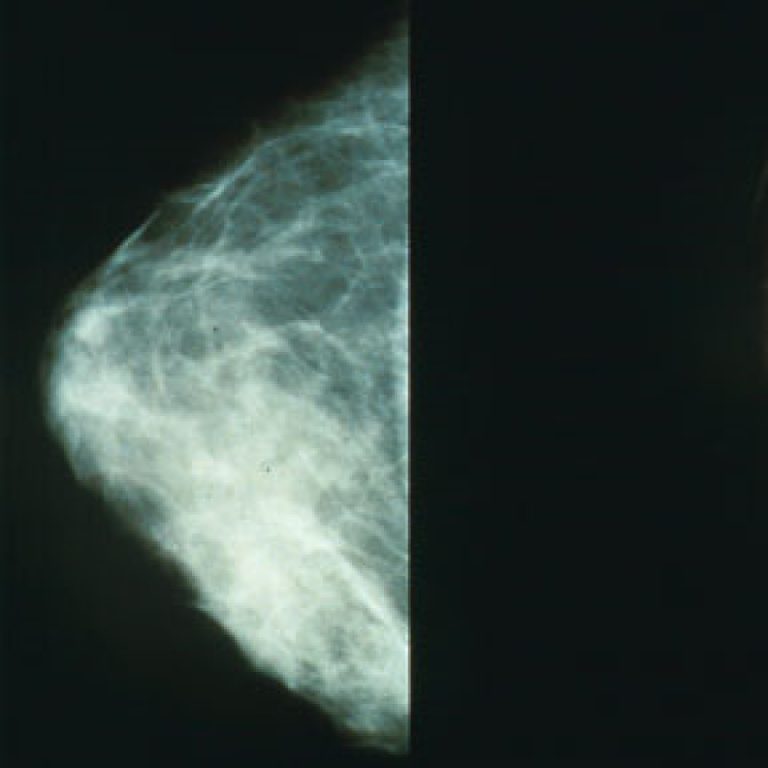
On Jun. 8, 2012, the U.S. Food and Drug Administration (FDA) announced that Genentech’s drug Perjeta (pertuzumab) was…

On Jun. 7, 2012, the FDA expanded licensure of PCV13 for prevention of pneumococcal disease to include adults…

On May 1, 2012, the U.S. Food and Drug Administration (FDA) approved new orphan drug Elelyso to treat…

On Feb. 17, 2012, the FDA announced Korlym (mifepristone) was approved to control high blood sugar levels (hyperglycemia)…

On Jan. 31, 2012, Vertex Pharmaceuticals announced that the U.S. Food and Drug Administration (FDA) had approved KALYDECOTM…

On Jan. 30, 2012, the U.S. Food and Drug Administration (FDA) approved Genentech’s Erivedge (vismodegib) capsule, the first…
On Dec. 30, 2011, Pfizer announced that the U.S. Food and Drug Administration (FDA) had granted approval of…
On Oct. 21, 2011, the Food and Drug Administration (FDA) announced it had approved revised prescribing information and…

On Aug. 19, 2011, the U.S. Food and Drug Administration (FDA) approved Seattle Genetics’ Adcetris (brentuximab vedotin), making…

On Aug. 17, 2011, Genentech announced that Elborafᆴ (vemurafenib) was approved by the FDA for the treatment of…
On Aug. 16, 2011, the U.S. Food and Drug Administration (FDA) approved vemurafenib tablets (Zelboraf, Hoffmann-LaRoche Inc.) for…

On Jul. 8, 2011, the FDA approved Boostrix (Tdap, GlaxoSmithKline) to prevent tetanus, diphtheria, and pertussis in older…

On Apr. 22, 2011, The U.S. Food and Drug Administration (FDA) approved the use of Menactra in children…
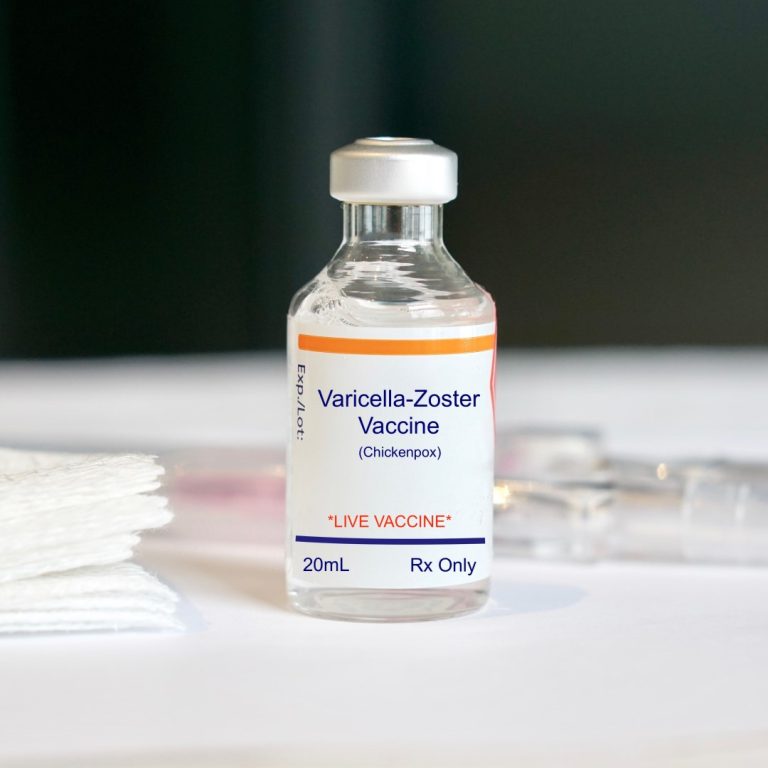
On Mar. 24, 2011, Zostavax, manufactured by Merck of Whitehouse Station, New Jersey, was approved by the U.S….

On Feb. 22, 2011, Foster City, Calif-based Gilead Sciences and Calistoga Pharmaceuticals announced Gilead’s acquisition of Calistoga for…

On Dec. 22, 2010, the FDA approved Gardasil HPV vaccine to include the indication for the prevention of…

On Apr. 29, 2010, the FDA approved sipuleucel-T (PROVENGE), made by the Dendreon Corp., an autologous cellular immunotherapy…

On Mar. 23, 2010, the U.S. Food and Drug Administration (FDA) recommended that physicians temporarily suspend use of…

On Feb. 24, 2010, a 13-valent pneumococcal conjugate vaccine (PCV13 [Prevnar 13, Wyeth Pharmaceuticals, a subsidiary of Pfizer])…
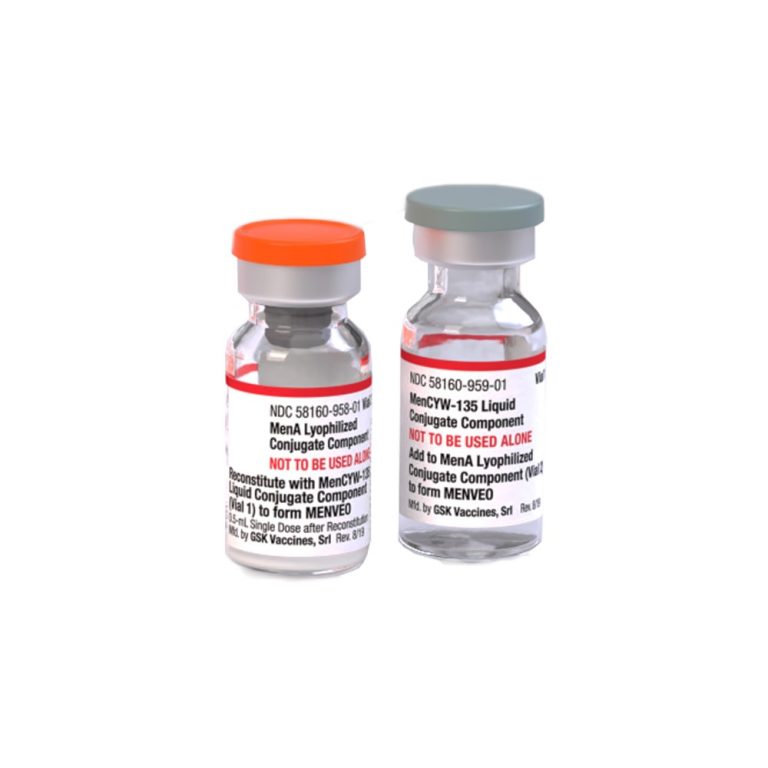
On Feb. 19, 2010, the U.S. Food and Drug Administration (FDA) announced it had licensed a quadrivalent meningococcal…
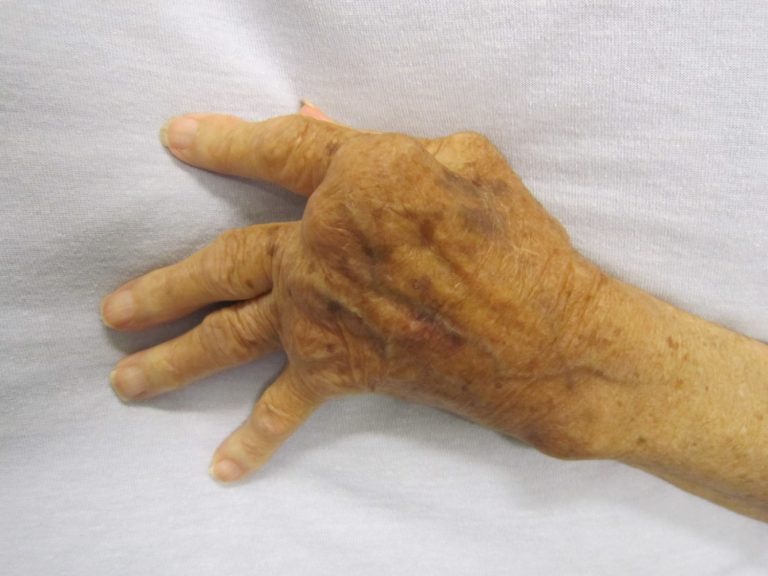
On Jan. 11, 2010, the U.S. Food and Drug Administration (FDA) approved Genentech’s tocilizumab (Actemra) for the treatment…