InBios received FDA EUA for its ZIKV Detect IgM Capture ELISA diagnostic test for antibodies in human serum
On Aug. 19, 2016, InBios announced that it had received an Emergency Use Authorization (EUA) from the U.S….

On Aug. 19, 2016, InBios announced that it had received an Emergency Use Authorization (EUA) from the U.S….

On Jul. 12, 2016, Pfizer announced that Prevnar 13ᆴ (Pneumococcal 13-valent Conjugate Vaccine [Diphtheria CRM197 Protein]) received FDA…

On Jun. 15, 2016, the FDA approved revisions in the package insert for YF-Vax to reflect changes to…
On Jun. 10, 2016, the U.S. Food and Drug Administration (FDA) approved Vaxchora, a vaccine for the prevention…
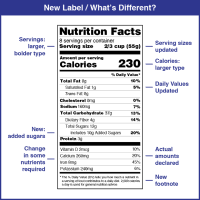
On May 27, 2016, the Food and Drug Administration (FDA) announced it had amended its labeling regulations for…

On Apr. 29, 2016, GlaxoSmithKline Biologicals in Research Triangle Park, North Carolina, received U.S. Food and Drug Administration…

On Jun. 14, 2016, the FDA approved changes to vaccine administrationᅠschedule for Trumenba vaccine produced by Wyeth Pharmaceuticals,…
On Apr. 11, 2016, the U.S. Food and Drug Administration (FDA) announced that it had granted accelerated approval…
On Dec. 14, 2015, the U.S. Food and Drug Administration (FDA) announced it had expanded Merck’s Gardasil 9…
On Dec. 11, 2015, Genentech announced that it’s drug Alecensa (alectinib) was approved by the U.S. Food and…

On Nov. 25, 2015, Seqirus announced that the U.S. Food and Drug Administration (FDA) had approved Fluad™ (Influenza…

On Nov. 19, 2015, AquaBounty Technologies announced the U.S. Food and Drug Administration (FDA) had approved the Company’s…

On Nov. 10, 2015, Genentech announed that its drug Cotellic (cobimetinib) was approved by the U.S. Food and…

On Sept. 4, 2015, the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices…

On Apr. 29, 2015, the U.S. Food and Drug Administration (FDA) announced it had approved Kybella, an injection…
On Mar. 24, 2015, the U.S. Food and Drug Administration (FDA) approved Sanofi’s Quadracel, a newᅠcombinationᅠDTaP+IPVᅠ vaccine for…
On Mar. 6, 2015, the The U.S. Food and Drug Administration (FDA) approved filgrastim-sndz (Zarxio, Sandoz, Inc.), the…

On Jan. 23, 2015, Novartis announced the US Food and Drug Administration (FDA) had granted accelerated approval of…

On Dec. 23, 2014, the U.S. Food and Drug Administration (FDA) approved a formulation of Novo Nordisk’s diabetes drug, liraglutide,…

On Dec. 22, 2014, BioCryst Pharmaceuticals announced the U.S. Food and Drug Administration (FDA) had approved RAPIVAB (peramivir…

On Dec. 11, 2014, Merck announced that the FDA had approved GARDASILᆴ9 (Human Papillomavirus 9-valent Vaccine, Recombinant), Merckメs…
On Dec. 12, 2014, Sanofi Pasteur announced the U.S. Food and Drug Administration (FDA) had approved the supplemental…

On Oct. 29, 2014, Pfizer announced the FDA has granted accelerated approval of TRUMENBAᆴ (meningococcal group B vaccine)…
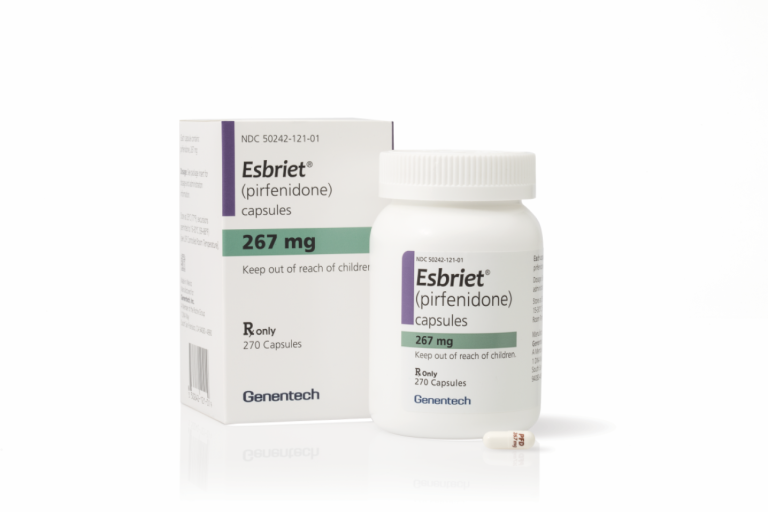
On Oct. 15, 2014, Genentech drug Esbriet (pirfenidone) was approved by the U.S. Food and Drug Administration (FDA)…
On Sept. 19, 2014, the Centers for Disease Control and Prevention”s (CDC) Advisory Committee on Immunization Practices (ACIP)…
On Aug. 14. 2014, Genentech announced that the U.S. Food and Drug Administration (FDA) had approved Avastin (bevacizumab)…

On Jun. 2, 2014, Omeros received U.S. Food and Drug Administration (FDA) approval of Omidria for use in…

On Apr. 1, 2014, Sanofi Pasteur announced the U.S. Food and Drug Administration (FDA) had expanded the approved…

On Nov. 13, 2013, Genentech announced that it’s drug Gazyva (obinutuzumab) was approved by the U.S. Food and…

On Aug. 16, 2013, the FDA extended FluLaval IIV (GlaxoSmithKline) age range to include children and teens age…