Roche announced FDA Emergency Use Authorization and availablabilityof cobas SARS-CoV-2 detection test
On Mar. 13, 2020, Roche announced the FDA has issued an Emergency Use Authorization for the cobasᆴ SARS-CoV-2…

On Mar. 13, 2020, Roche announced the FDA has issued an Emergency Use Authorization for the cobasᆴ SARS-CoV-2…

On Mar. 13, 2020, Thermo Fisher Scientific announced the FDA has issued an emergency use authorization (EUA) for…

On Mar. 9, 2020, the FDA approved Boehringer Ingelheim’s Ofev (nintedanib) oral capsules to treat patients with chronic…

On Mar. 6, 2020, the FDA approved Isturisa (osilodrostat) oral tablets for adults with Cushing’s disease who either…

On Mar. 4, 2020, the UW Medicine Clinical Virology Lab announced it had received U.S. Food and Drug…

On Mar. 2, 2020, Mateon Therapeutics reported the company had been evaluating its therapeutic and AI platforms to…

On Feb. 29, 2020, the University of Washington (UW) Medicine Clinical Virology Lab announced it had received U.S….

On Feb. 28, 2020, the FDA approved an application for the first generic of Daraprim (pyrimethamine) tablets for…

On Feb. 24, 2020, Innovation Pharmaannounced the Company submitted a Material Transfer Agreement (MTA) with a leading U.S.-based…

On Feb. 22, 2020, a federal judge from the Southern District of New York ruled drug companies, device…

On Feb. 21, 2020, the U.S. Food and Drug Administration (FDA) authorized marketing of the first test to…

On Feb. 21, 2020, the U.S. Food and Drug Administration (FDA) approved NEXLETOL (bempedoic acid) tablet, an oral,…

On Feb. 18, 2020, Innovation Pharma announced the Company was exploring its lead defensin mimetic drug candidate, Brilacidin,…
On Jan. 14, 2020, Valneva announced that the U.S. Department of Defense (DoD) had exercised an option to…

On Jan. 8, 2020, the U.S. Food and Drug Administration (FDA) approved patient-specific airway stents developed by Cleveland…

On Dec. 20, 2019, the U.S. Food and Drug Administration (FDA) granted accelerated approval to Daiichi Sankyo’s Enhertu…

On Dec. 18, 2019, Seagen and Astellas Pharma announced the U.S. Food and Drug Administration (FDA) had granted…

On Dec. 17, 2019, the University of Alabama at Birmingham Comprehensive Cancer Center (UAB) announced that Gregory Friedman,…
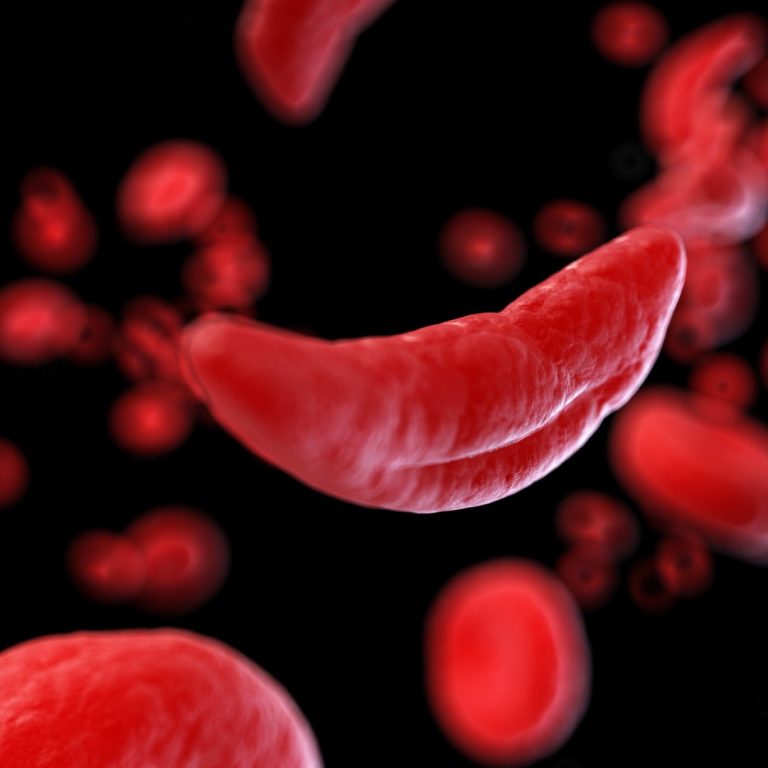
On Dec. 2, 2019, the U.S. Food and Drug Administration (FDA) announced it had approved the drug crizanlizumab…
On Nov. 22, 2019, the Centers for Disease Control and Prevention”s (CDC) Advisory Committee on Immunization Practices (ACIP)…
On Nov. 4, 2019, the U.S. Food and Drug Administration (FDA) approved Fluzone High-Dose Quadrivalent (Sanofi Pasteur) for…

On Oct. 17, 2019, Genentech announced the U.S. Food and Drug Administration (FDA) had approved Xofluza (Baloxavir Marboxil),…
On Sept. 24, 2019, the U.S. Food and Drug Administration announced approval of Jynneos Smallpox and Monkeypox Vaccine,…

On Jun. 10, 2019, Genentech drug Polivy (polatuzumab vedotin-piiq) announced the U.S. Food and Drug Administration (FDA) had…

On May 24, 2019, La Jolla, CA-based AveXis, a subsidiary of Novartis, received U.S. Food and Drug Administration…

On May 23, 2019, the U.S. Food and Drug Administration (FDA) announced that it had authorized InBios’ marketing…

On Apr. 24, 2019, GSK announced $100 million of new investment in its manufacturing site in Hamilton, Montana…

On Apr. 9 2019, Amgen announced U.S. Food and Drug Administration (FDA) approval of EVENITY (romosozumab-aqqg) for the…

In Mar. 29, 2019, the Centers for Disease Control and Prevention (CDC) issued new guidance to clinicians for…
On Feb. 28, 2019, the U.S. Food and Drug Administration (FDA) approved trastuzumab and hyaluronidase-oysk injection,for subcutaneous use…