Childhood exposure to bacterial toxin may be triggering colorectal cancer epidemic among the young
On Apr. 23, 2025, researchers from the Wellcome Sanger Institute, University of California San Diego and their collaborators…

On Apr. 23, 2025, researchers from the Wellcome Sanger Institute, University of California San Diego and their collaborators…

On Apr. 21, 2025, the National Institutes of Health (NIH) reported that overall death rates from cancer declined steadily among…

On Apr. 17, 2025, research-integrity analysts are warning that ‘journal snatchers’ — companies that acquire scholarly journals from…

On Apr. 17, 2025, the U.S. Centers for Disease Control and Prevention reported results from a study by…
On Apr. 17, 2025, researchers from the University of of Pittsburgh announced they have produced the most detailed…
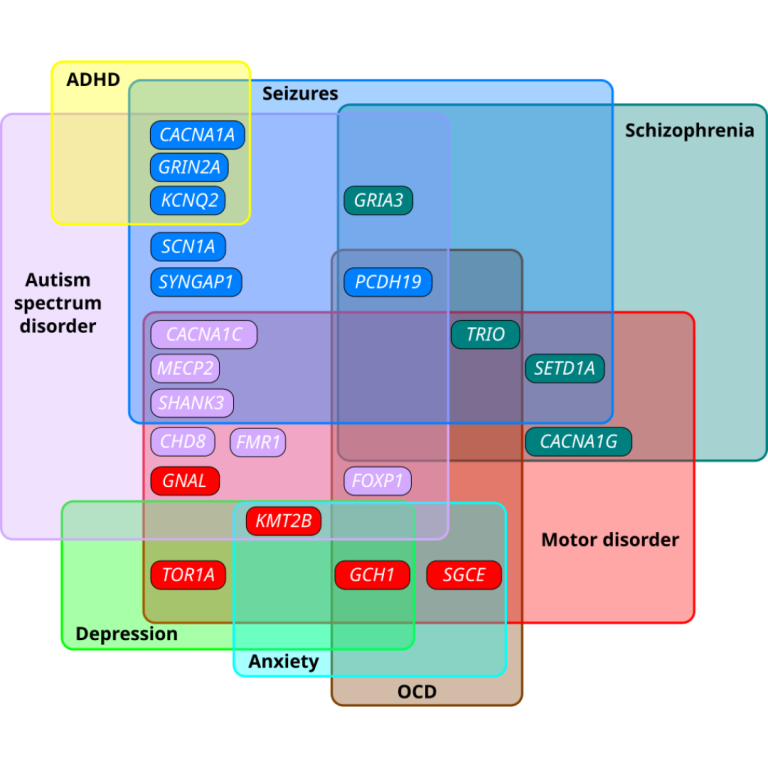
On Apr. 14, 2025, a new University of Southern California (USC) study suggests that gut imbalances in children…
On Apr. 14, 2025, a research team at the Institute for Basic Science (IBS) in Korea announced they…

On Apr. 11, 2025, the UK National Institute for Health and Care Excellence (NICE) recommended capivasertib (also called…
On Apr. 10, 2025, the U.S. Food and Drug Administration announced it is taking a groundbreaking step to…

On Apr. 9, 2025, Roswell Park Comprehensive Cancer Center achieved textbook outcomes for 90% of 150 consecutive robot-assisted,…

On Apr. 7, 2025, in a landmark study, researchers at University of Nebraska Medical Center (UNMC) announced they…

On Apr. 4, 2025, a 3-year-old girl from the western state of Durango is Mexico’s first confirmed human…

On Apr. 3, 2025, a team led by researchers at Baylor College of Medicine and the Jan and…

On Apr. 3, 2025, a Stanford-led study has found that the gene CXCL12 is connected to the posterior…

On Apr. 3, 2025, The International Journal of Neonatal Screening, an international, peer-reviewed journal focused on newborn screening…

On Apr. 3, 2025, a collaborative research led by investigators at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center…

On Apr. 2, 2025, Northwestern University engineers announced they have developed a pacemaker so tiny that it can…

On Apr. 1, 2025, Mountain snowpacks accumulate snow throughout the winter, building up stores of water that will…
On Mar. 28, 2025, the U.S. Food and Drug Administration (FDA) announced approval of Alnylam Pharmaceuticals Qfitlia™ (fitusiran),…

On Mar. 27, 2025, Utah become the first state to ban fluoride in public drinking water, over opposition…
On Mar. 27, 2025, the California Institute for Regenerative Medicine (CIRM), one of the world’s largest institutions dedicated…

On Mar. 26, 2025, researchers announced they have discovered a new antibiotic molecule that targets a broad range…

On Mar. 26, 2025, the Biotechnology Innovation Organization (BIO) released results from a membership survey that underscores the…

On Mar. 25, 2025, Merck announced it has signed a licensing agreement for a heart disease drug with…

On Mar. 25, 2025, GSK announced that the US Food and Drug Administration (FDA) has approved Blujepa (gepotidacin)…

On Mar. 24, 2025, LifeScienceHistory.com published the cartoon “Milestones in Fluoride” illustrating the facts behind the naturally occurring…

On Mar. 24, 2025, scientists at the UCLA Health Jonsson Comprehensive Cancer Center and the University of Toronto…

On Mar. 23, 2025, 23andMe announced that it has initiated voluntary Chapter 11 proceedings in the U.S. Bankruptcy…

On Mar. 20, 2025, Alnylam Pharmaceuticals announced the U.S. Food and Drug Administration (FDA) approval of the supplemental…
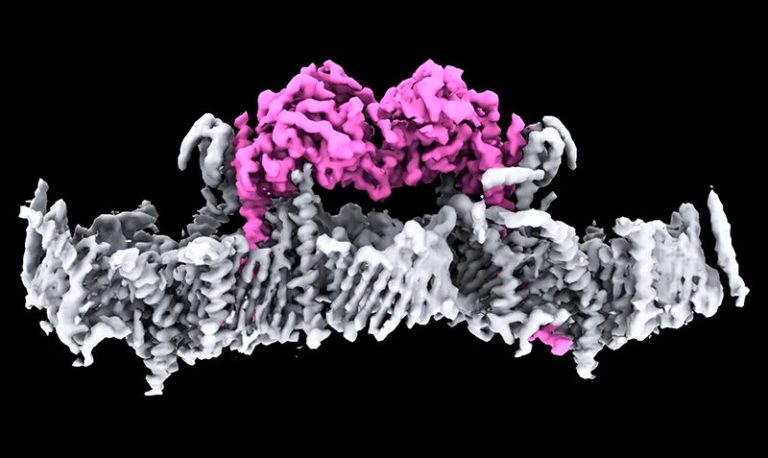
On Mar. 14, 2025, researchers from the Walter and Eliza Hall Institute of Medical Research (WEHI) announced they…