The Openness Promotes Effectiveness in our National Government Act of 2007 amended the Freedom of Information Act
On Dec. 31, 2007, the U.S. Congress passed the Openness Promotes Effectiveness in our National Government Act, or…

On Dec. 31, 2007, the U.S. Congress passed the Openness Promotes Effectiveness in our National Government Act, or…

On Dec. 19, 2007, the Human Microbiome Project (HMP), a $150 million initiative, was established by the National…

On Dec. 18, 2007, World Autism Awareness Day (WAAD) shines a bright light on autism as a growing…

On Dec. 6, 2007, the Cancer Therapy & Research Center became part of the UT Health Science Center…

On Nov. 27, 2007, a new National Cancer Institute (NCI) model for calculating invasive breast cancer risk, called…

On Nov. 19, 2007, the University of California at San Francisco Cancer (UCSF) Research Institute was renamed the…

On Oct. 30, 2007, the Barnes-Jewish Hospital (BJC) Institute of Health at Washington University was established, to house…

On Oct. 29, 2007, the International HapMap Consortium announced it had published an analyses of its second-generation map…

On Oct. 9, 2007, the David H. Koch Institute for Integrative Cancer Research at MIT was established with…

On Sept. 24, 2007, David H. Murdock donated $35 million to Duke University to fund the Measurement to…

On Sept. 15, 1997, Alain Carpentier at Hospital European Goerges Pompidou in Paris and Albert Starr at Providence…

On Sept. 14, 2007, the U.S. Food and Drug Administration (FDA) announced it had approved Eli Lilly’s osteoporosis…
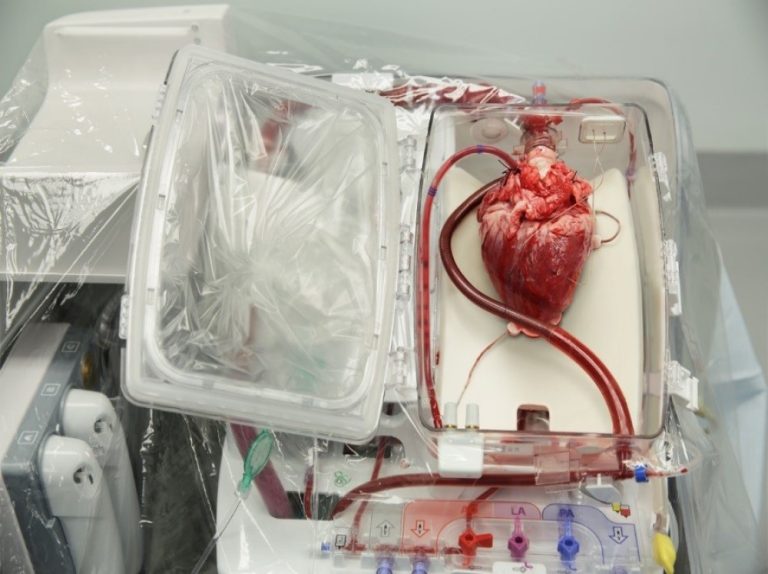
On Aug. 16. 2007, surgeons at the University of Washington (UW) Medical Center performed Seattle’s first adult heart-lung…

On Jun. 7, 2007, years before the COVID-19 pandemic swept across the globe, a group of about 100…

On Apr. 18, 2007, a study led by the University of Texas M.D. Anderson Cancer Center reported that…
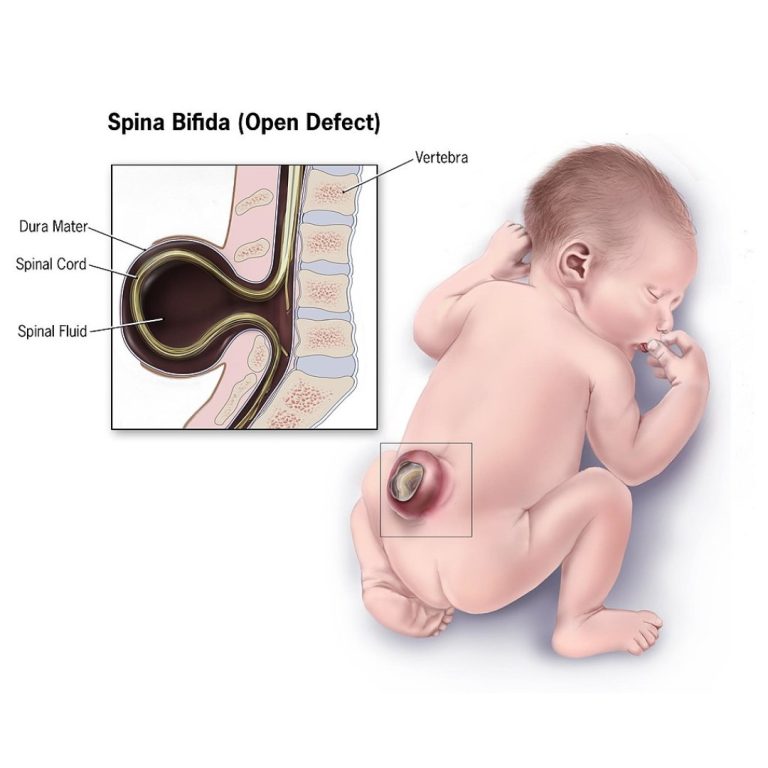
On Apr. 5, 2007, Dr. Philippe Gros and his team of McGill University researchers identified a gene that…
On Apr. 1, 2007, National Cancer Institute (NCI) researchers reported that a variation in a portion of DNA…

On Mar. 28, 2007, Magnetic Resonance Imaging (MRI) scans of women who were diagnosed with cancer in one…

On Mar. 20, 2007, the National Institute on Alcohol Abuse and Alcoholism (NIAAA) announced a five-year, $24 million…

On Feb. 8, 2007, Oregon Health & Science University (OHSU) and the OHSU Foundation announced the largest outright gift…

On Feb. 1, 2007, the Centers for Disease Control and Prevention (CDC) released its long-awaited disease containment strategy…

In 2007, Cocrystal Discovery, now known as Cocrystal Pharma, was founded in Bothell as a pharmaceutical company with…

On Apr. 25, 2007, Jubilant Organosys, Ltd. announced it had acquired Spokane-based Hollister-Stier for $122.5 million. The plant…

In 2007, West Campus, located on 136 acres 7 miles west of downtown New Haven, was acquired. West…

In 2007, the U.S. Food and Drug Administration (FDA) approved Elelyso (taliglucerase alfa) for long-term enzyme replacement therapy…

In 2007, Nancy Wexler, PhD, received the Benjamin Franklin Medal in Life Science for her discovery of the…
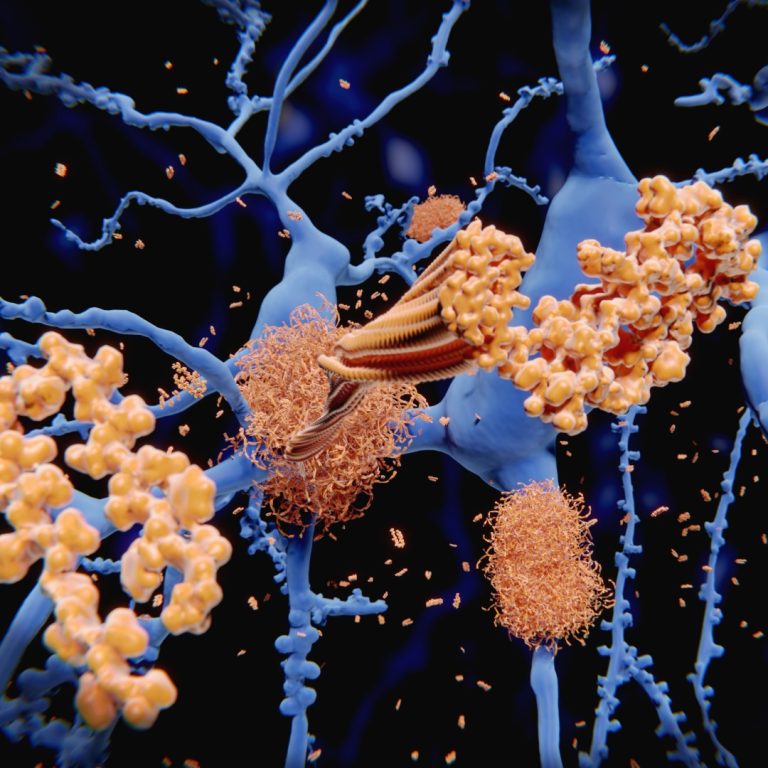
In 2007, Dr. Jordan Tang, led a team of scientists at Oklahoma Medical Research Foundation (OMRF) that identified…

In 2007, Texas Biomed geneticists announced their discovery that a particular gene, VNN1, plays a major role in…

In 2007, the Center for Clinical and Translational Research (CCTR) was founded at Virginia Commonwealth University to enhance…
On Nov. 16, 2006, the U.S. Food and Drug Administration (FDA) approved Genentech’s trastuzumab (Herceptin) for use with…