WHO Reports Diphtheria outbreak in African Region
On Nov. 21, 2025, the World Health Organization (WHO) reported that from 1 January to 2 November 2025,…

On Nov. 21, 2025, the World Health Organization (WHO) reported that from 1 January to 2 November 2025,…

On Nov. 20, 2025, the National Institutes of Health (NIH) has canceled funding for at least 383 clinical…
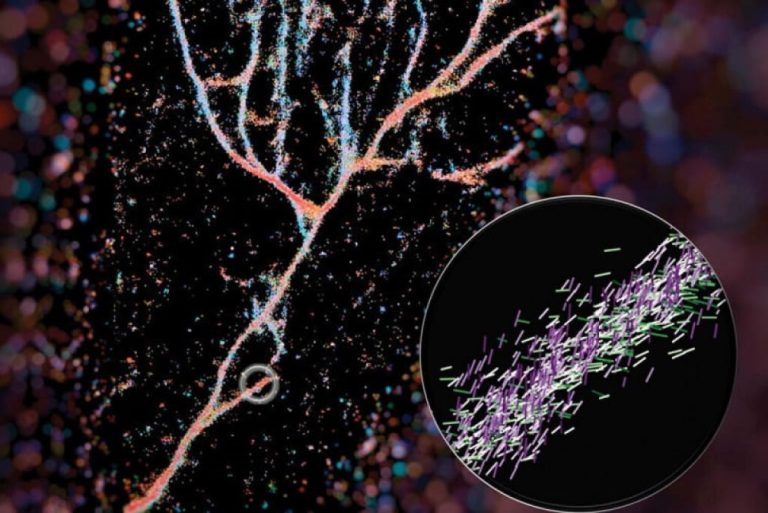
On Nov. 19, 2025, a study by researchers from Nanyang Technological University (NTU) Singapore announced they had discovered…

On Nov. 18, 2025, Stanford Medicine researchers reported that a combination blood stem cell and pancreatic islet cell…

On Nov. 17, 2025, Sandoz announced that TYRUKO® (natalizumab-sztn) is available to patients in the US. Developed by…
On Nov. 17, 2025, in a major step forward for cancer care, researchers at ChristianaCare’s Gene Editing Institute…

On Nov. 12, 2025, a study led by the Hebrew University of Jerusalem, and the Laboratory of Entomology…
On Nov. 10, 2025, a study led by the University of Liverpool reported that existing evidence does not…

On Nov. 6, 2025, the Institute for Health Metrics and Evaluation (IHME) at the University of Washington School…

On Nov. 6, 2025, the World Health Organization (WHO) released a technical report, The future of paediatric clinical trials…

On Nov. 5, 2025, a large study of adults with type 2 diabetes showed that those who consistently…
On Nov. 5, 2025, an Osaka Metropolitan University team has used stem cells extracted from adipose, the body’s…
On Nov. 5, 2025, MIT researchers announced they have developed a new 3D human brain tissue platform, the…

On Nov. 4, 2025, the U.S. Food and Drug Administration (FDA) approved revumenib, a first-in-class oral menin inhibitor,…

On Nov. 4, 2025, a team of scientists led by the University of Washington announced findings published this…

On Nov. 3, 2025, United Therapeutics, a public benefit corporation, announced the first clinical xenotransplantation in its EXPAND…

On Oct. 30, 2025, the California Institute for Regenerative Medicine (CIRM) approved awarding $27 million through three grants…
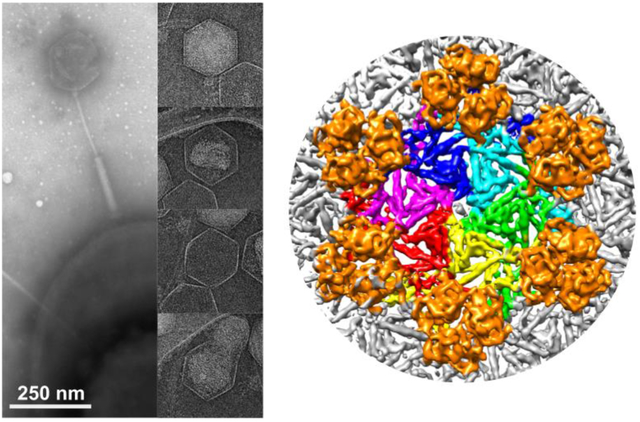
On Oct. 29, 2025, University of North Carolina at Charlotte (UNC) researchers leading a multidisciplinary team across four…

On Oct. 27, 2025, researchers from Johns Hopkins Medicine and the Johns Hopkins Bloomberg School of Public Health…

On Oct. 27, 2025, researchers at University of California San Diego School of Medicine (UC San Diego) have…

On. Oct. 23, 2025, Air pollution continues to exacerbate global health, including noncommunicable diseases and dementia, according to…

On Oct. 17, 2025, Chinese biotech Hansoh Pharma said it had signed a license agreement worth up to…

On Oct. 16, 2025, the Food and Drug Administration (FDA) announced the first round of experimental drugs that…
On Oct. 13, 2025, researchers from Texas A&M University announced they have identified two estrogens, estradiol and estriol,…

On Oct. 13, 2025, the U.S. Food and Drug Administration (FDA) announces it has cleared the Elecsys pTau181…

On Oct. 10, 2025, Bristol Myers Squibb (BMS) and Orbital Therapeutics announced a definitive agreement under which BMS…
On Oct. 9, 2025, Beicheng Sun at Anhui Medical University in China and his colleagues have reported the…
On Oct. 8, 2025, scientists at the University of East Anglia and Oxford Biodynamics announced they have developed a…

On Oct. 6, 2025, the World Health Organization (WHO) reported world is smoking less, but the tobacco epidemic…
On Oct. 6, 2025, Researchers at the University of Bath, in collaboration with the Universities of Oxford and…