WHO and Medicines Patent Pool announce sublicensing agreement for rapid diagnostic test technology
On May 9, 2025, The World Health Organization (WHO) and Medicines Patent Pool (MPP) announced a sublicensing agreement…

On May 9, 2025, The World Health Organization (WHO) and Medicines Patent Pool (MPP) announced a sublicensing agreement…
On May 9, 2025, the Texas Department of State Health Services (DSHS) reported 709 cases of measles have…

On May 9, 2025, Kodak announced it was readying to open its new $20 million manufacturing facility at…

On May 9, 2025, Teal Health® announced the Food and Drug Administration’s (FDA) approval of the Teal Wand™,…
On May 7, 2025, the New Mexico Department of Health (NMDOH) confirmed the first measles cases the first…

On May 7, 2025, Gilead Sciences announced $11 billion in new planned investment in the U.S. to add…
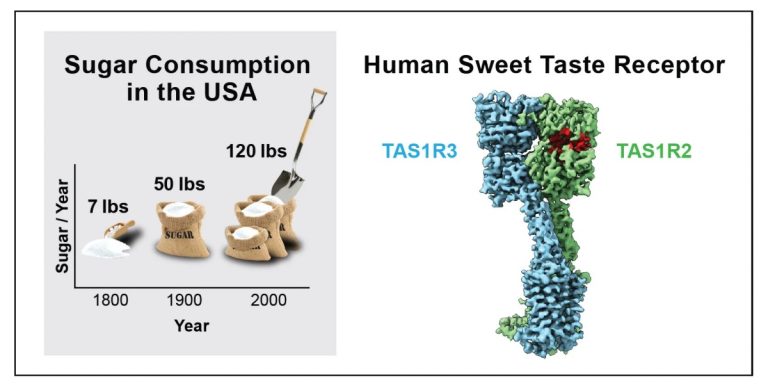
On May 7, 2025, Howard Hughes Medical Institute Investigator Charles Zuker and members of his Columbia University lab…

On May 7, 2025, a team of Mayo Clinic researchers has developed an artificial intelligence (AI) system that…

On May 7, 2025, researchers at the University College London (UCL) and King’s College London announced that an…

On May 6, 2025, UT Southwestern Medical Center and Children’s Health℠ announced a historic nine-figure grant from the Moody Foundation…

On May 5, 2025, The European Union (EU) and France announced half a billion euros worth of incentives…

On May 2, 2025, the Centers for Disease Control reported twelve pediatric deaths associated with seasonal influenza virus…

On May 2, 2025, the Texas Department of State Health Services (DSHS) reported 683 cases of measles have…

On May 1, 2025, the U.S. National Institutes of Health (NIH), the world’s largest funder of biomedical research,…
On May 1, 2025, the U.S. Centers for Disease Control and Prevention (CDC) announced that hospitals are no…

On Apr. 30, 2025, the U.S. Food and Drug Administration (FDA) approved the use of a gene-editing technology…
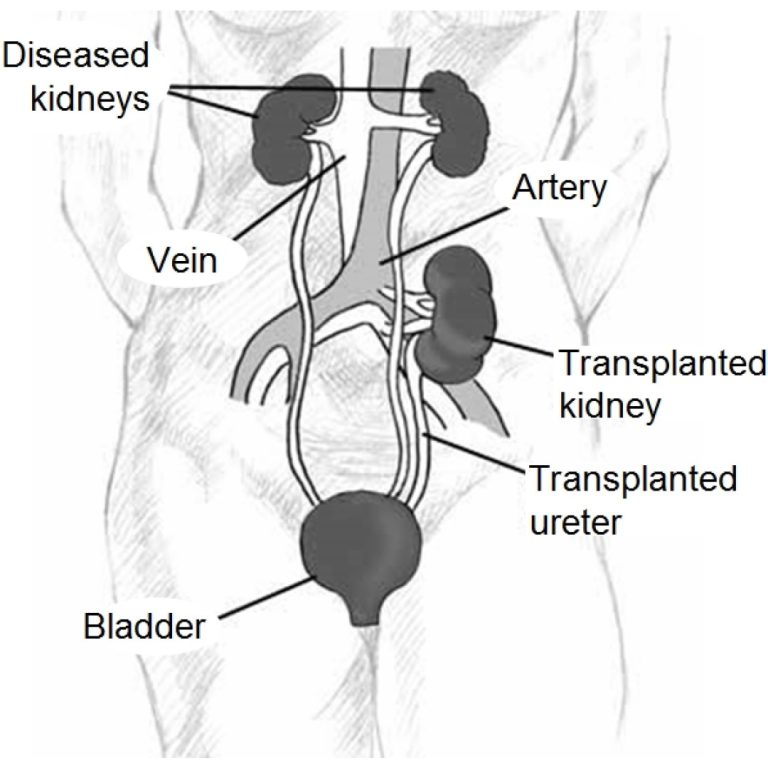
On Apr. 28, 2025, researchers at King’s College London, the University of Bristol and Great Ormond Street Hospital…

On Apr. 28, 2025, the U.S. Centers for Disease Control and Prevention (CDC) announced it had awarded a more than $3…
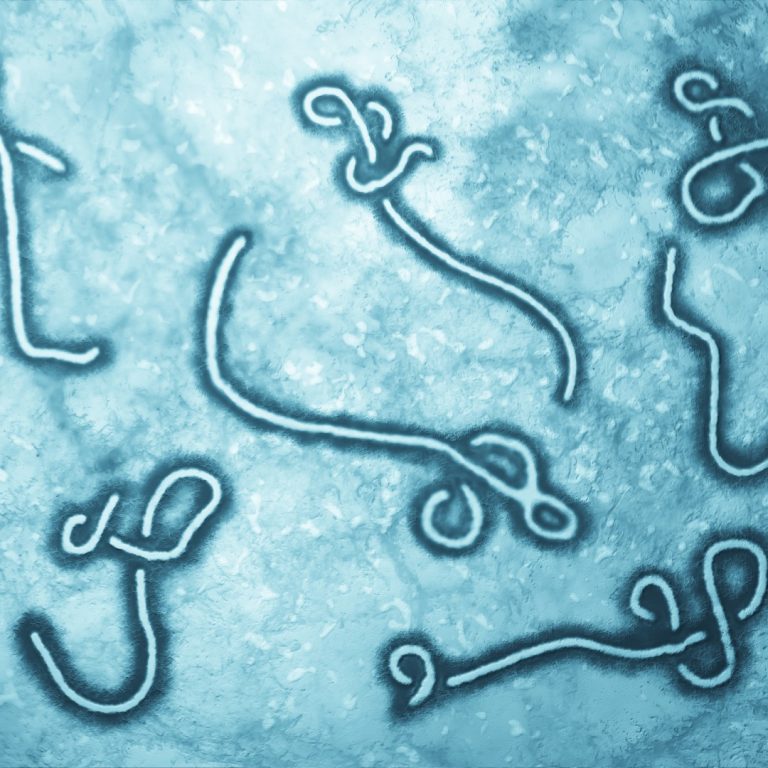
On Apr. 26, 2025, the World Health Organization (WHO) reported that Uganda has declared the end of the…
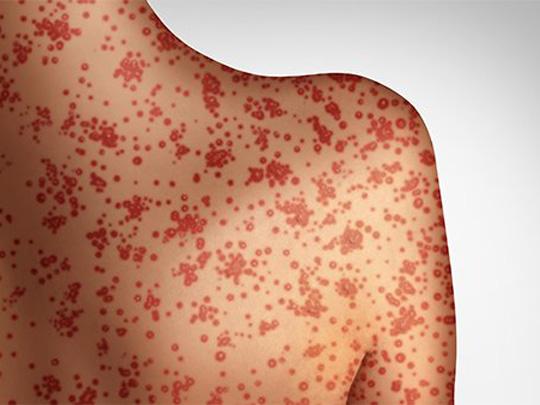
On Apr. 25, 2025, the Texas Department of State Health Services (DSHS) reported that 646 cases of measles…

On Apr. 25, 2025, University of Washington announced that the Memory & Brain Wellness Center at Harborview Medical…

On Apr. 24, 2025, Thermo Fisher Scientific announced it will will invest an additional $2 billion in the…

On Apr. 23, 2025, researchers from the Wellcome Sanger Institute, University of California San Diego and their collaborators…
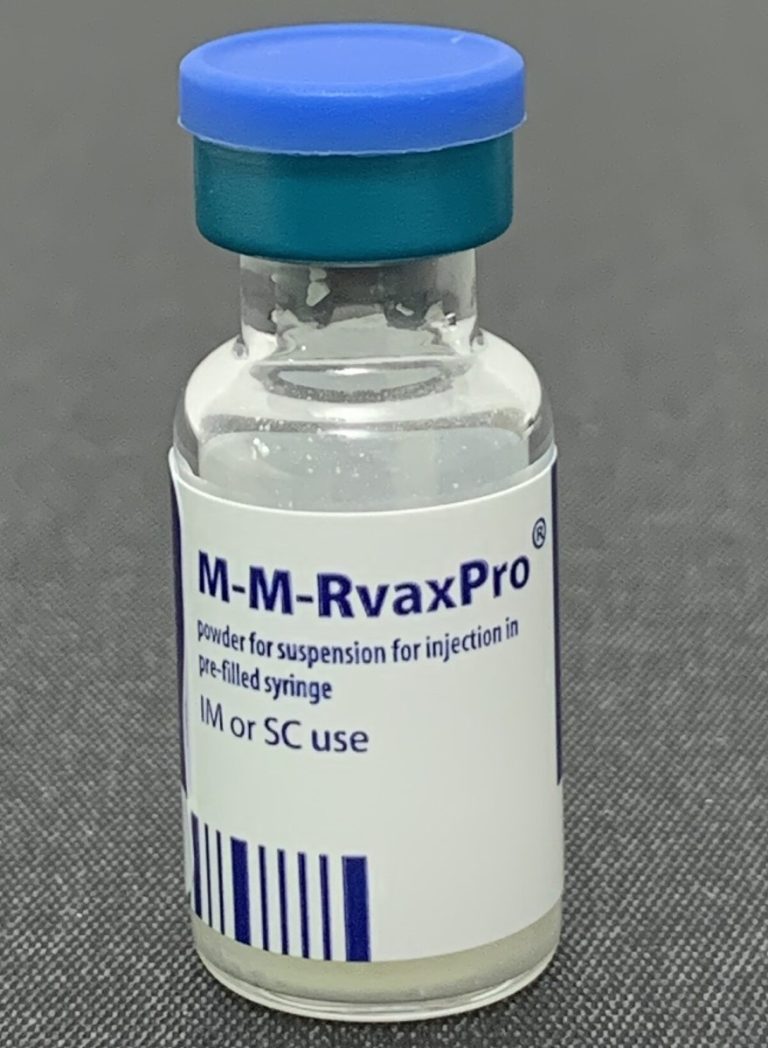
On Apr. 22, 2025, Seattle & King County was notified of a confirmed measles case in a King…

On Apr. 22, 2025, Roche announced that it will invest USD $50 billion into the United States in…

On Apr. 22, 2025, Syngenta announced that its next-generation insecticide Sovrenta® has received pre-qualification by World Health Organization…

On Apr. 21, 2025, the National Institutes of Health (NIH) reported that overall death rates from cancer declined steadily among…

On Apr. 21, 2025, LifeScienceHistory.com published the cartoon: “The National Institutes of Health (NIH) and U.S. Healthcare in…

On Apr. 19, 2025, the Virginia Department of Health (VDH) reported the state’s first measles case of the…
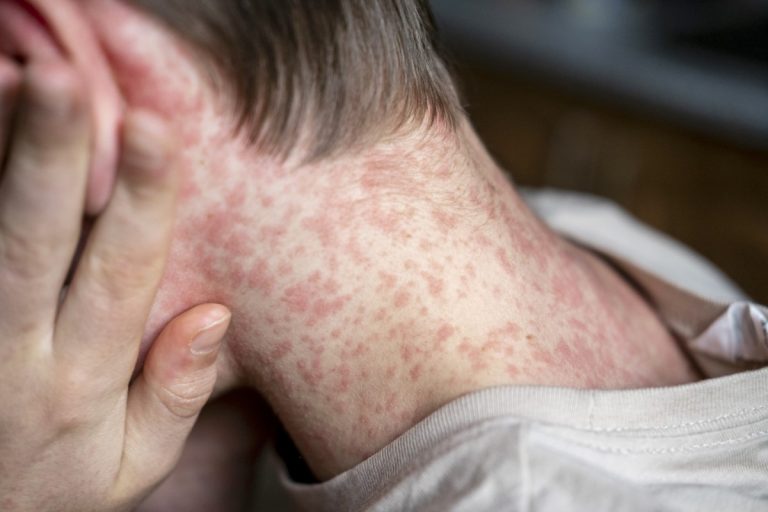
On Apr. 18, 2025, the Texas Department of State Health Services (DSHS) reported that 597 cases of measles…