Testing with combined biopsy method improves prostate cancer diagnosis in NCI study
On Mar. 4, 2020, a method of testing for prostate cancer developed at the National Cancer Institute (NCI)…
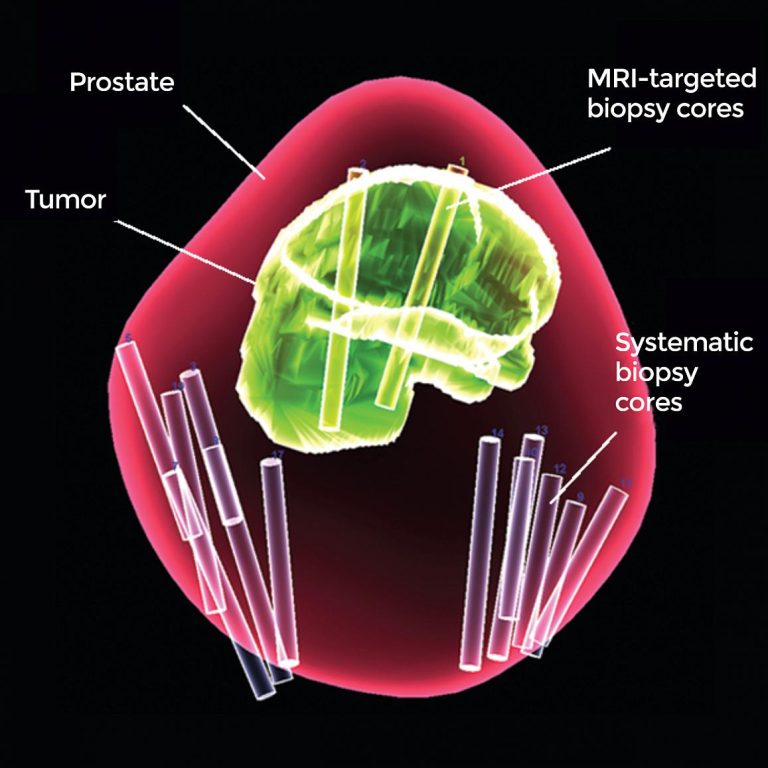
On Mar. 4, 2020, a method of testing for prostate cancer developed at the National Cancer Institute (NCI)…
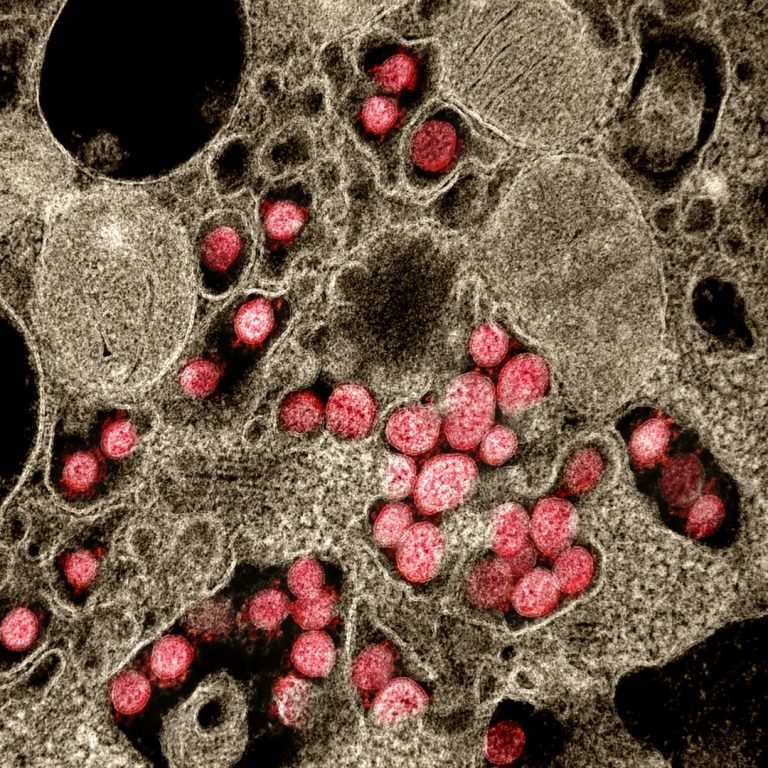
On Mar. 4, 2020, Luminex announced they were working on multiple solutions to augment their NxTAG and ARIES…

On Mar. 20, 2020, BioReference Labs, an OPKO Health company, announced a collaboration with the State of New…
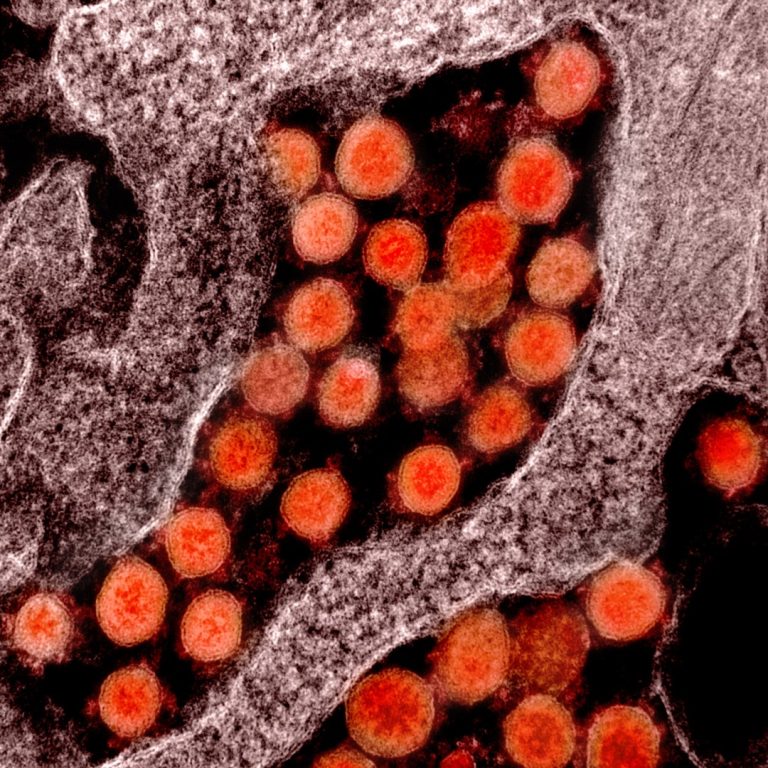
On Mar. 2, 2020, Boston’s top infectious disease researchers, including scientists from Boston University’s National Emerging Infectious Diseases…

On Mar. 1, 2020, the United Nations Humanitarian Chief Mark Lowcock released US$15 million from the Central Emergency…

On Feb. 29, 2020, the University of Washington (UW) Medicine Clinical Virology Lab announced it had received U.S….
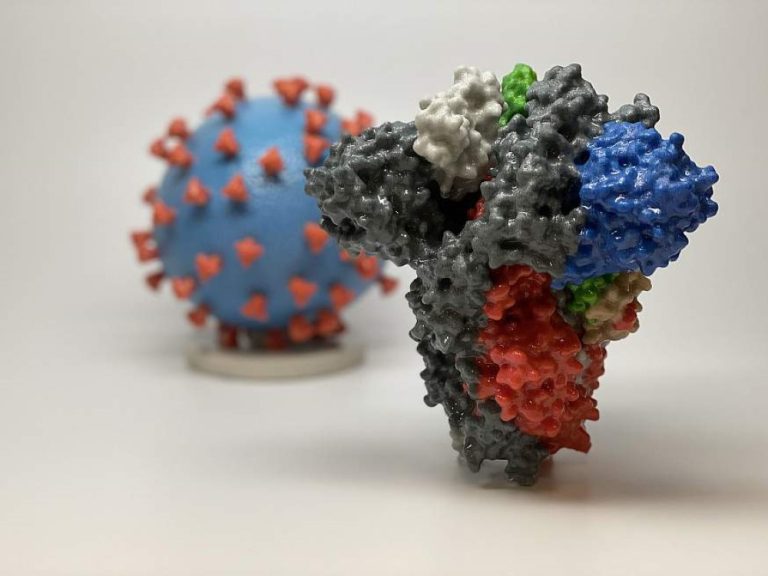
On Feb. 28, 2020, commentary appeared in The New England Journal of Medicine that discussed the emergence and…

On Feb. 27, 2020, Innovation Pharma announced the Company had signed a Material Transfer Agreement (MTA) with one…
On Feb. 25, 2020, the U. S. National Institutes of Health (NIH) announced it had launched a $1…

On Feb. 24, 2020, researchers at National Institute of Allergy and Infectious Disease’s Rocky Mountain Laboratories in Hamilton,…
On Feb. 24, 2020, Scientists at Texas Biomed began work on the novel coronavirus known as SARS-CoV-2. A…

On Feb. 21, 2020, the U.S. Food and Drug Administration (FDA) authorized marketing of the first test to…

On Feb. 20, 2020, Tim Boyle, CEO of Columbia Sportswear, and his wife, Mary, made a $10 million…

On Feb. 19, 2020, NIAID scientists working with investigators from the University of Texas at Austin identified the…
On Feb. 19, 2020, the Cancer Prevention & Research Institute of Texas (CPRIT) announced it had awarded 55…
On Feb. 10, 2020, the World Health Organization (WHO) and the Foundation for Innovative New Diagnostics (FIND) announced…

On Feb. 5, 2020, to fight further spread of the new coronavirus (2019-nCoV) outbreak in China and globally,…
On Feb. 5, 2020, an international team announced that it had completed the most comprehensive study of whole…
On Jan. 30, 2020, the Centers for Disease Control and Prevention (CDC) confirmed that the 2019 Novel Coronavirus…

On Jan. 30, 2020, the University of Washington (UW) announced that a new center was being created with…

On Jan. 28, 2020, the Director-General of the World Health Organization (WHO), Dr. Tedros Adhanom Ghebreyesus, met President…

On Jan. 28, 2020, AbCellera confirmed that it had mobilized its pandemic response platform against the ongoing outbreak…

On Jan. 27, 2020, the Jackson Laboratory announced it was one of the founding partners of the Roux…

On Jan. 27, 2020, the Cancer Prevention and Research Institute of Texas (CPRIT) announced it had recruited its…

On Jan. 26, 2020, the Bill & Melinda Gates Foundation announced that it had immediately committed $10 million…

On Jan. 20, 2020, deCODE genetics reported a genome-wide association meta-analysis of 69,189 cases and 702,199 controls from…

On Jan. 13, 2020, a new Cleveland Clinic study uncovered a genetic anomaly associated with poor response to…

On Jan. 13, 2020, Vanderbilt University Medical Center researcher Ela Knapik, MD and her colleagues announced they had…
On Jan. 10, 2020, Mayo Clinic researchers reported that they had discovered a molecular switch for repairing central…
On Jan. 9, 2020, the World Health Organization announced that a novel coronavirus had been identified as the…