BD to invest $12 billion in pre-fillable syringe manufacturing capacity over next four years
On Dec. 2, 2020, BD (Becton, Dickinson) announced plans to invest approximately $1.2 billion over a 4-year period…
On Dec. 2, 2020, BD (Becton, Dickinson) announced plans to invest approximately $1.2 billion over a 4-year period…

On Dec. 2, 2020, the University of Oregon opened the Phil and Penny Knight Campus for Accelerating Scientific…

On Nov. 30, 2020, Moderna announced that the primary efficacy analysis of the Phase 3 study of mRNA-1273…

On Nov. 20, 2020, the ‘European Food Safety Authority reported that within the past month more than 300…

On Nov. 19, 2020, University of Oxford researchers announced that research into the HIV-1 virus had shed light…

On Nov. 19, 2020, Cue Health announced that, as of November 9, the U.S. Department of Health and…

On Nov. 19, 2020, XBiotech announced data for its breakthrough candidate therapy for treating infections of influenza and…

On Nov. 19, 2020, Dallas-based company, Worlds Inc., the U.S. Air Force and Texas A&M University announced a…

On Nov. 18, 2020, Oregon Health & Science University (OHSU) announced an initiative that will attempt to discern…
On Nov. 16, 2020, bioMerieux announced the expansion of its ARGENE range for the detection of SARS-CoV-2. As…

On Nov. 16, 2020, the UK Department for Health and Social Care Testing Innovation Fund announced £12.2M funding…

On Nov. 16, 2020, Tonix Pharmaceuticals announced preliminary results following vaccination of non-human primates with TNX-1800 (modified horsepox…

On Nov. 16, 2020, Aegis Sciences announced that it had launched a combined test for SARS-CoV-2 and influenza…

On Nov. 13, 2020, a study from Washington University School of Medicine in St. Louis and St. Jude…

On Nov. 12, 2020, AstraZeneca announced that the CALAVI Phase II trials for Calquence (acalabrutinib) in patients hospitalised…
On Nov. 12, 2020, Wren Laboratories announced that it had been granted an emergency use authorization by the…
On Nov. 10, 2020, BD (Becton, Dickinson) announced its rapid, point-of-care, SARS-CoV-2 antigen test for use on the…

On Nov. 9, 2020, the Fred Hutchinson Cancer Research Center announced the start of volunteer enrollment for a…

On Nov. 6, 2020, Mesa Biotech announced it had been awarded a contract up to $13 million from…

On Nov. 5, 2020, Denmark announced strict lockdown rules in the north of the country after authorities discovered…

On Nov. 4, 2020, McGill University Professor William Foulkes awarded 2020 Wilder-Penfield Prize for research in the genetics…
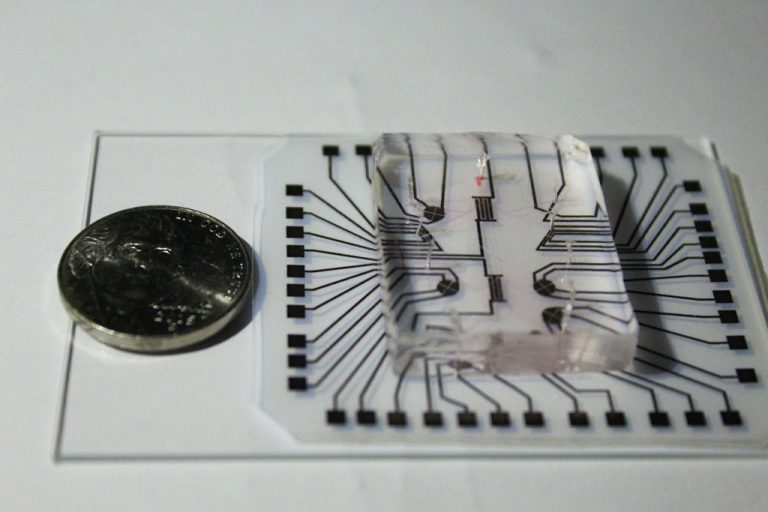
On Nov. 4, 2020, using the cutting-edge genetic editing technique known as CRISP ‘lab on a chip’ technology,…

On Nov. 3, 2020, OraSure Technologies announced its DNA Genotek subsidiary has received Emergency Use Authorization from the…

On Nov. 1, 2020, the U.S. Department of Defense announced the start of rapid, on-site COVID-19 testing for…

On Oct. 30, 2020, the U.S. Department of Health and Human Services (HHS) and the U.S. Department of…
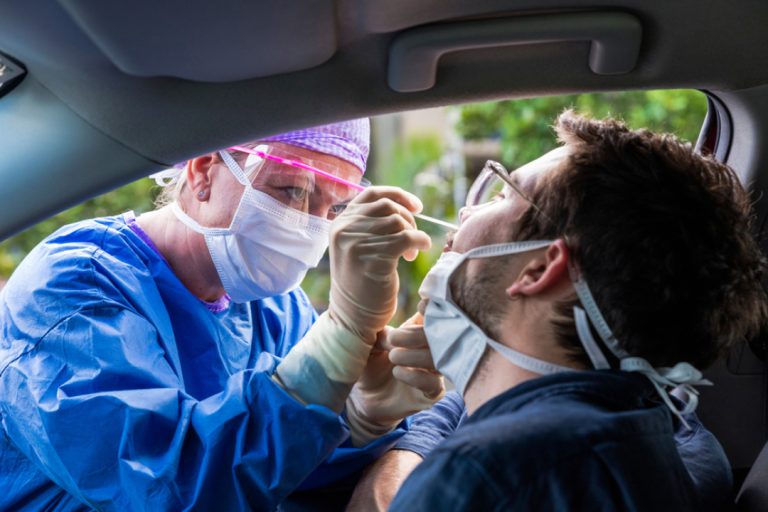
On Oct. 29, 2020, Mammoth Biosciences announced that it had signed agreements with MilliporeSigma and Hamilton Company targeting…

On Oct. 29, 2020, the University of California, Berkeley (UC Berkeley) announced that it had launched a new…
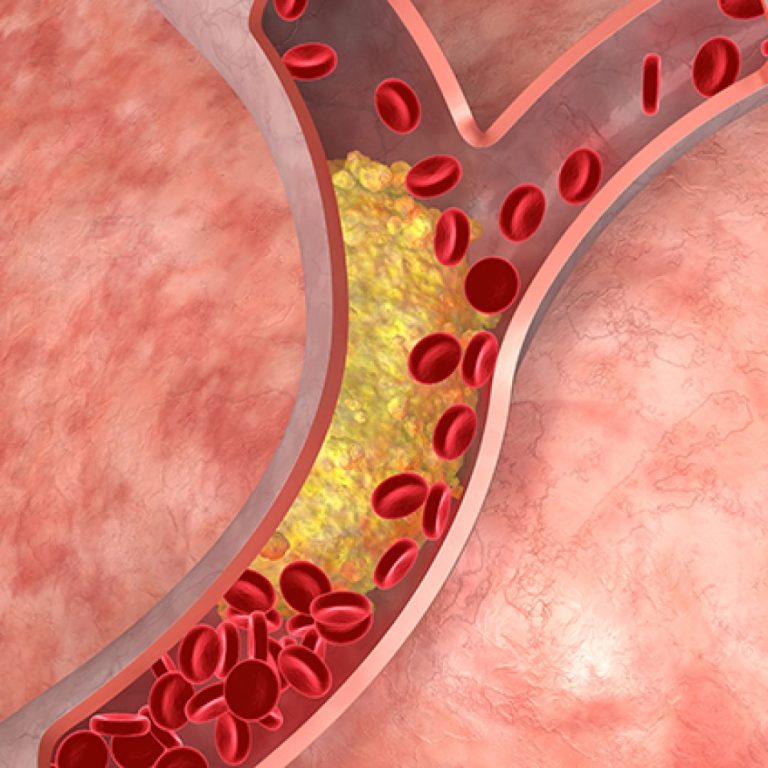
On Oct. 28, 2020, University of Texas (UT) Southwestern scientists reported that an X-ray test commonly used to…

On Oct. 27, 2020, a National Institutes of Health (NIH) study of 5,000 women found that approximately 1…

On Oct. 27, 2020, researchers from the National Institutes of Health (NIH) announced they had discovered a new…