Vaccine doses allocated to African countries hardest hit by mpox surge
On Nov. 6, 2024, the World Health Organization announced that the Access and Allocation Mechanism (AAM) for mpox…

On Nov. 6, 2024, the World Health Organization announced that the Access and Allocation Mechanism (AAM) for mpox…

On Nov. 6, 2024, researchers from the Pasteur Institute in Cambodia published a detailed genetic analysis of the…
On Nov. 6, 2024, data from the VENUS (Vaccine Effectiveness, Networking, and Universal Safety) study in Japan revealed…
On Nov. 6, 2024, researchers from Emory University, in collaboration with scientists at Gladstone Institutes and University of…

On Nov. 6, 2024, The United Kingdom’s Chief Veterinary Officers was urging all bird keepers to follow scrupulous…

On Nov. 5, 2024, Scripps research scientists reported they have shown that Alzheimer’s and alcohol use disorder (AUD)…
On Nov. 5, 2024, a cross-sectional study of data for 36 cancer types from 185 countries and territories…
On Nov. 4, 2024, the U.S. Food and Drug Administration (FDA), in collaboration with the Environmental Protection Agency…
On Nov. 4, 2024, a team of University of Wisconsin–Madison researchers warned that artificial intelligence tools gaining popularity…

On Nov. 4, 2024, a study conducted by researchers from the CUNY Graduate School of Public Health and…

On Nov. 3, 2024, the world’s largest biodiversity summit, known as COP16, concluded with several landmark decisions, including…

On Nov. 1, 2024, researchers reported results from a study that show a low-sugar diet during pregnancy and…
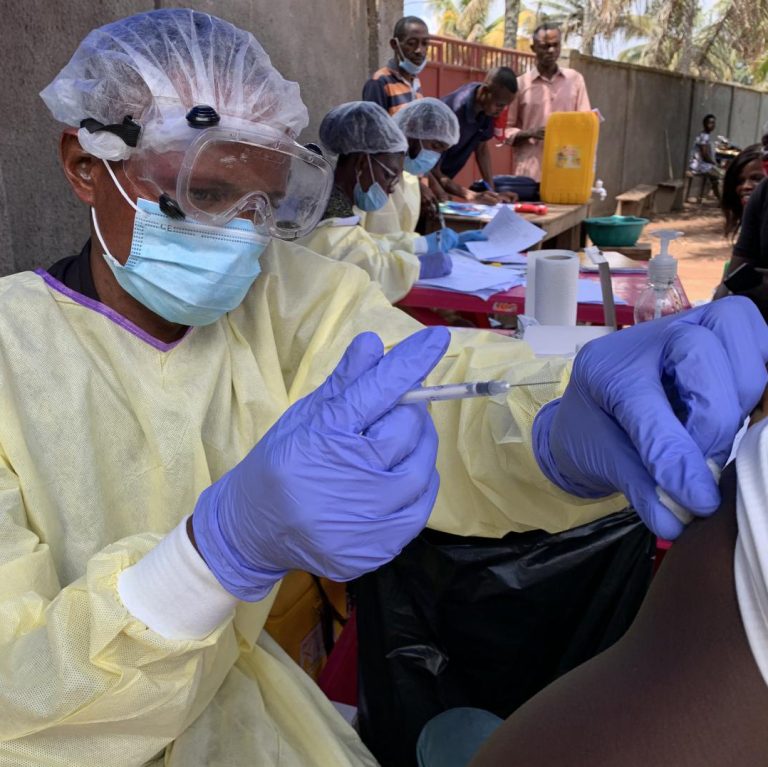
On Nov. 1, 2024, the World Health Organization reported that as of October 31, 66 confirmed cases, including…

On Oct. 31, 2024, the Oregon Department of Agriculture reported that bird flu cases are on the rise,…

On Oct. 31, 2024, a University of Southern California (USC) study involving 8,500 children from across the country…
On Oct. 30, 2024, ten studies from the Human Tumor Atlas Network (HTAN), a National Institutes of Health…
On Oct. 30, 2024, an international research team led by the University of Bonn reported results of a…

On Oct. 30, 2024, the U.S. Centers for Disease Control (CDC) reported that fresh, slivered onions served on…

On Oct. 29, 2024, a study led by Andrei Gudkov, PhD, DSci, at Roswell Park Comprehensive Cancer Center support…

On Oct. 29, 2024, University of Florida scientist John Lednicky, Ph.D., an expert in viruses, working with colleagues,…
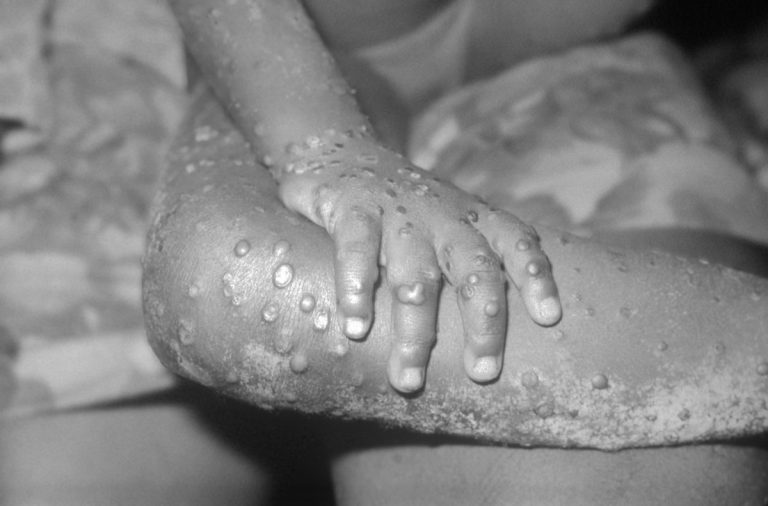
On Oct. 29, 2024, the World Health Organization (WHO) announced that it had, in collaboration with Member States,…
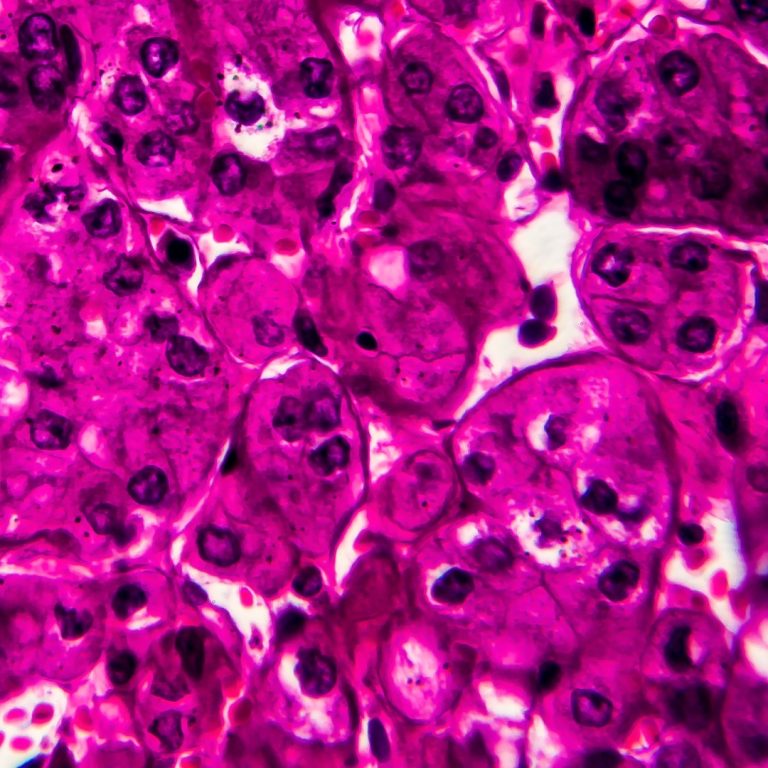
On Oct. 29, 2024, a study led by investigators from the UCLA Health Jonsson Comprehensive Cancer Center (UCLA…
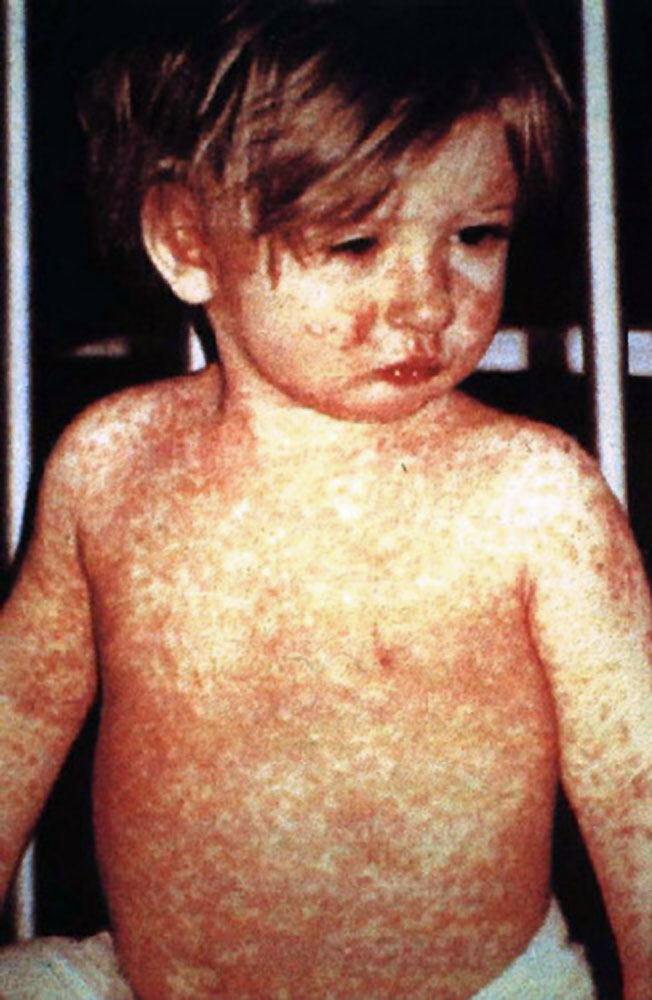
On Oct. 29, 2024, the BC Centre for Disease Control announced that travellers were alerted to potential exposure…

On Oct. 28, 2024, the Johns Hopkins University and Johns Hopkins Medicine, together with descendants of Henrietta Lacks,…
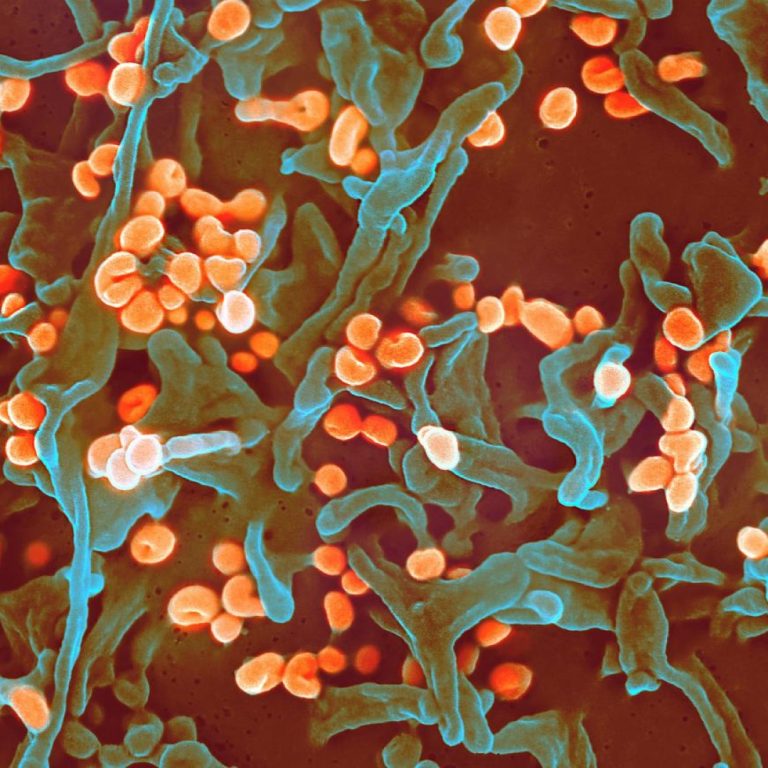
On Oct. 28, 2024, the Iowa Department of Health and Human Services (HHS) confirmed the death of a…
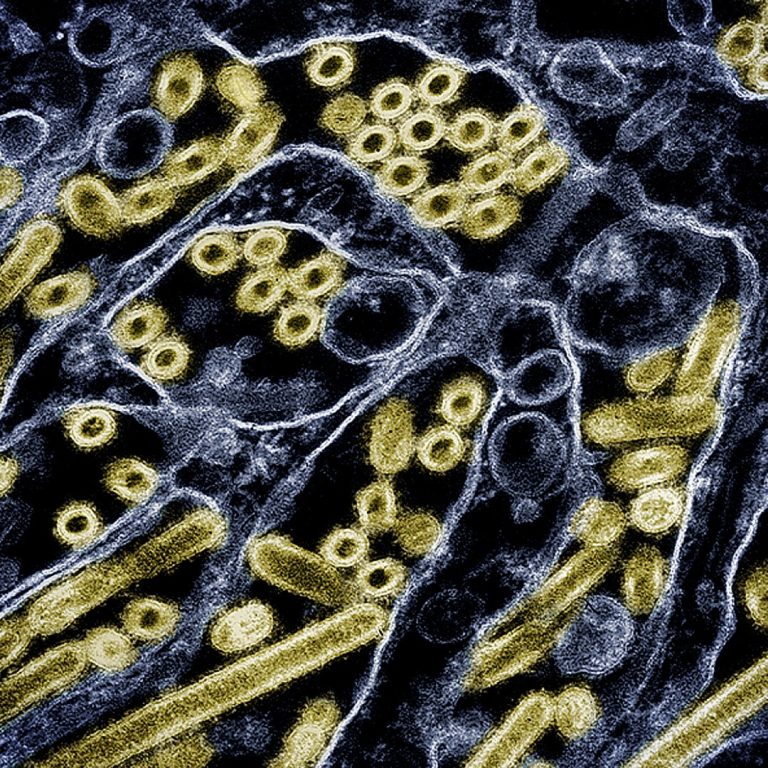
On Oct. 28, 2024, University of Wisconsin–Madison researchers reported that a strain of H5N1 avian influenza virus found…

On Oct. 25, 2024, the World Health Organization reported that as of October 24, a total of 64…
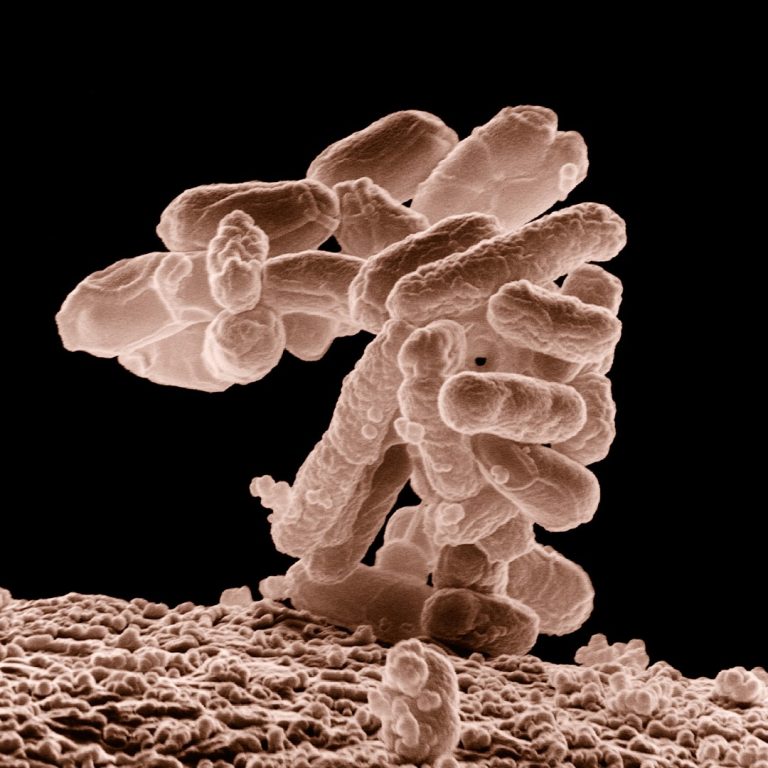
On Oct. 25, 2024, the Centers for Disease Control and Prevention (CDC) reported illnesses have been reported in…

On Oct. 24, 2024, the Centers for Disease Control and Prevention (CDC) confirmed that two people who had…

On Oct. 23, 2024, the University of Rochester announced that the Saunders Foundation, led by University Trustee Emeritus…