Research shows path to easier diagnosis of mitochondrial disorders
On Feb. 11, 2025, in study of mitochondrial disease at the University of Alberta researcher revealed an easier…
On Feb. 11, 2025, in study of mitochondrial disease at the University of Alberta researcher revealed an easier…

On Feb. 11, 2025, the Arizona Department of Agriculture (AZDA) reported working in conjunction with the United States…
On Feb. 10, 2025, scientists announced identifying genetic variants that could have helped a man to avoid dementia…

On Feb. 8, 2025, the United States Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS)…

On Feb. 5, 2025, the Spokane Regional Health District announced the first confirmed death from pertussis (whooping cough)…

On Feb. 4, 2025, an international research teams led by Hong Kong University of Science and Technology (HKUST)…
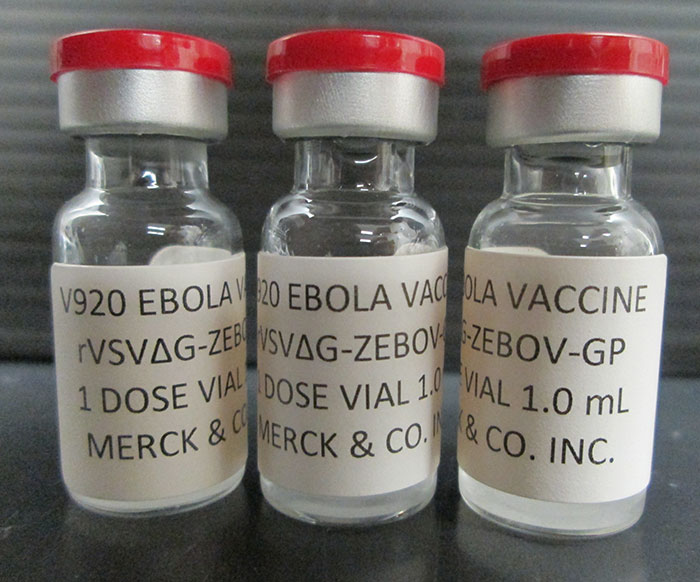
On Feb. 3, 2025, in a global first, Uganda’s Ministry of Health, the World Health Organization (WHO) and…
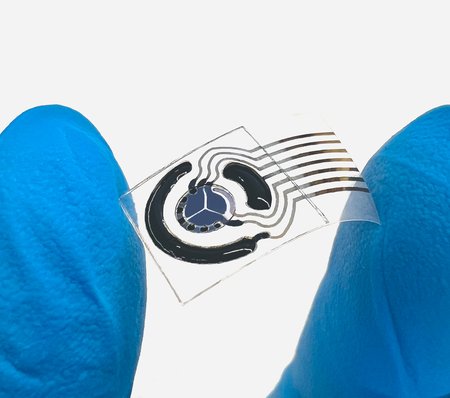
On Feb. 3, 2025, a team of Caltech engineers announced they have developed a technique for inkjet printing…
On Feb. 3, 2025, a team of researchers co-led by TGen, part of City of Hope announced creating…
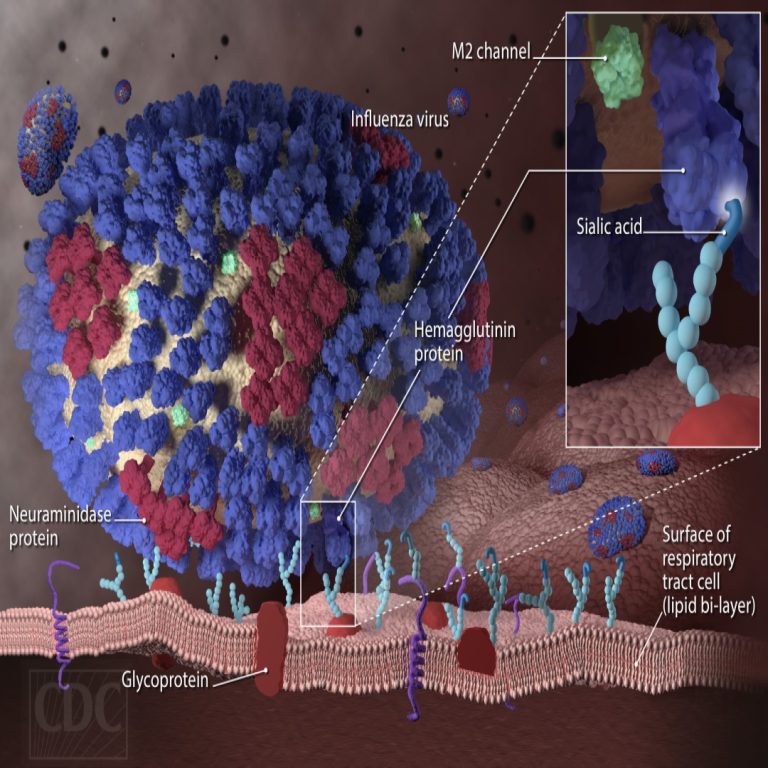
On Jan. 31, 2025, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) confirmed…
On Jan. 31, 2025, the Kansas Department of Health and Environment (KDHE) reported that there were 67 confirmed…

On Jan. 30, 2025, the California Institute for Regenerative Medicine (CIRM) announced it has awarded nearly $100 million…

On Jan. 30, 2025, the World Health Organization (WHO) announced it was mobilizing efforts to support national health…

On Jan. 30, 2025, the World Health Organization (WHO) congratulated Niger for having met the criteria for onchocerciasis…
On Jan. 29, 2025, researchers at The University of Queensland announced they have identified the first henipavirus in…

On Jan. 27, 2025, researchers at the Broad Institute of MIT and Harvard, along with collaborators at Calico…

On Jan. 27, 2024, the World Organisation for Animal Health (WOAH) reported that highly pathogenic avian influenza (HPAI)…
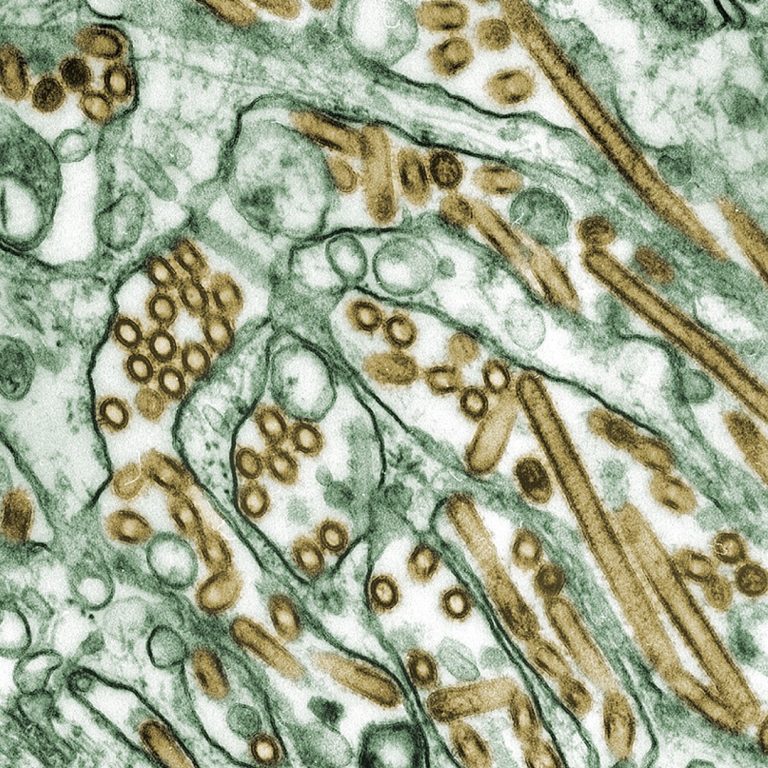
On Jan. 27, 2025, researchers reported that the H5N1 virus is adapting to new mammalian hosts, raising the…

Jan. 24, 2025, the U.S. Food and Drug Administration (FDA) pulled draft guidance from its website requiring companies…
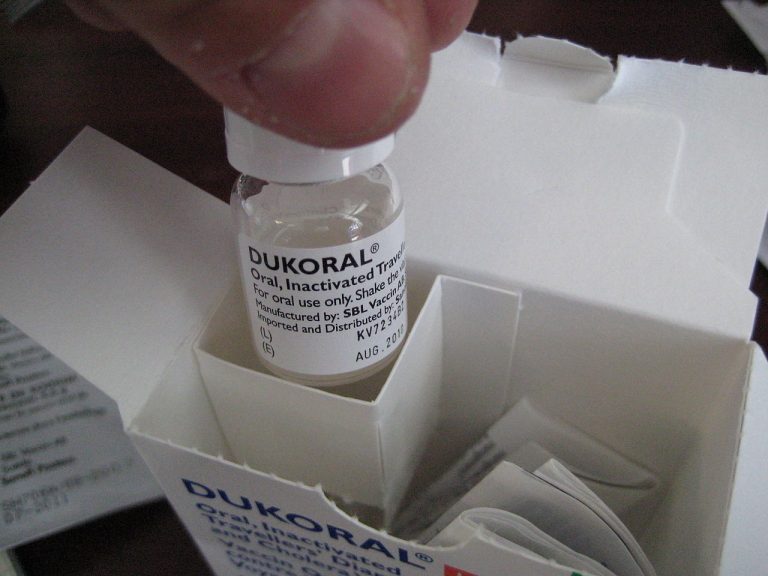
On Jan. 24, 2025, the World Health Organization (WHO) reported that from January 1 to December, 29, 2024,…

On Jan. 24, 2025, researchers from the Veterans Affairs Portland Health Care System in Oregon reported results from…

On Jan. 24, 2025, The South Dakota Department of Health reported the death of a child due to…

On Jan. 23, 2025, the World Health Organization (WHO) announced that following a nearly century-long effort, Georgia has…

On Jan. 22, 2025, researchers from the University of Virginia School of Medicine reported revealing the fingerprints of…
On Jan. 22, 2025, researchers at Dana-Farber Cancer Institute announced they have developed a breakthrough method to detect…
On Jan. 22, 2025, scientists at deCODE genetics/Amgen announced they have constructed a complete map of how human…

On Jan. 22, 2025, the U.S. Department of Agriculture (USDA) Animal and Plant Health Inspection Service (APHIS) has…

On Jan. 22, 2025, researchers have identified chronic wasting disease (CWD) prions in raw, cooked, and cured meat…
On Jan. 20, 2025, the World Health Organization (WHO) announced that Tanzania has confirmed an outbreak of Marburg…
On Jan. 20, 2025, a large international 68-country survey of 71,922 respondents found that in most countries, most…