Moderna announced supply agreement with Qatar for mRNA vaccine against COVID-19
On Oct. 26, 2020, Moderna announced a supply agreement with the Ministry of Public Health of Qatar for…

On Oct. 26, 2020, Moderna announced a supply agreement with the Ministry of Public Health of Qatar for…

On Oct. 26, 2020, Todos Medical announced the completion of clinical validation and CLIA certification for MOTO+PARA’s mobile…

On Oct. 26, 2020, PerkinElmer announced that its PKamp Respiratory SARS-CoV-2 RT-PCR Panel had received clearance to be…

On Oct. 26, 2020, ContraFect announced that it had initiated an expanded access program to provide exebacase for…

On Oct. 26, 2020, Todos Medical announced the completion of the instrument validation in its mobile lab division….

On Oct. 26, 2020, Taconic Biosciences announced immediate humanized ACE2 mouse model availability for COVID-19 research. The SARS-CoV-2…

On Oct. 26, 2020, Membrion announced a breakthrough in the fight against the COVID-19 virus: a molecular coating…

On Oct. 23, 2020, Entos Pharmaceuticals announced it was receiving advisory services and funding of up to $5…
On Oct. 23, 2020, Johnson & Johnson announced that it was preparing to resume recruitment in the pivotal…

On Oct. 22, 2020, Amyris and the Infectious Disease Research Institute (IDRI) announced the signing of a Collaboration…

On Oct. 22, 2020, Moderna announced that it had completed enrollment of 30,000 participants for the Phase 3…

On Oct. 22, 2020, Todos Medical announced that it had entered into an exclusive supply agreement with U.S….

On Oct. 22, 2020, Gilead Sciences announced that the FDA had approved the antiviral drug Veklury (remdesivir) for…

On Oct. 22, 2020, the FDA approved the antiviral drug Veklury (remdesivir) for use in adult and pediatric…

On Oct. 22, 2020, Roche and Atea Pharmaceuticals announced they had joined forces in the fight against COVID-19…

On Oct. 22, 2020, Oxford Immunotec announced that it had been selected to provide T cell testing to…
On Oct. 22, 2020, the World Health Organization (WHO) and the Wikimedia Foundation announced a collaboration to expand…
On Oct. 22, 2020, IAVI, a nonprofit scientific research organization dedicated to addressing urgent, unmet global health challenges,…
On Oct. 21, 2020, ViiV Healthcare, majority owned by GSK, with Pfizer and Shionogi Limited as shareholders, announced…

On Oct. 21, 2020, ImmunityBio and NantKwest announced that the first patient had been dosed in the Phase…

On Oct. 21, 2020, CerTest Biotec, along with BD (Becton, Dickinson), announced that the VIASURE SARS-CoV-2 (N1 +…

On Oct. 20, 2020, the NIH announced that the researchers had completed the Adaptive COVID-19 Treatment Trial (ACTT-1),…

On Oct. 20, 2020, BioMedomics announced that the National Institute for Food and Drug Surveillance (INVIMA) had provided…
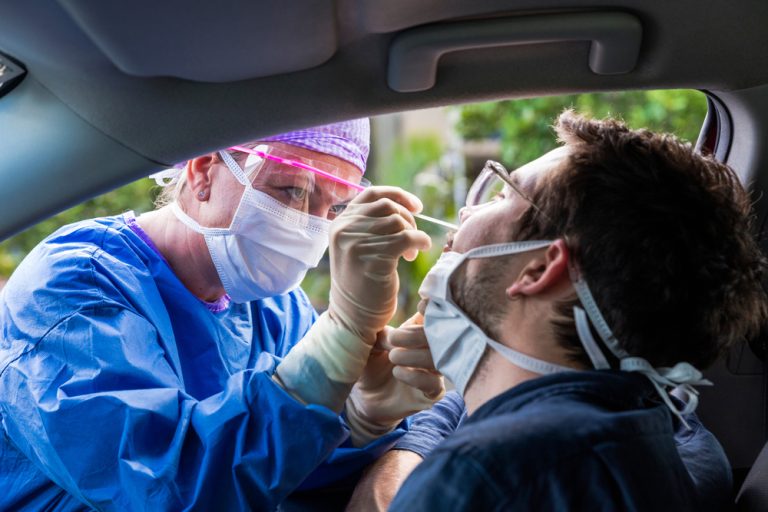
On Oct. 20, 2020, BioReference Laboratories, an OPKO Health company, announced that it was accepting specimens for a…

On Oct. 20, 2020, the European Investment Bank (EIB) and Immunic announced the signing of a タ24.5 million…

On Oct. 20, 2020, researchers at Oregon Health & Science University (OHSU) and Oregon State University (OSU) reported…

On Oct. 19, 2020, LabCorp announced a test that provided a quantitative measurement of an individualメs SARS-CoV-2 IgG…

On Oct. 19, 2020, ICON announced that it had been re-selected by the Biomedical Advanced Research and Development…

On Oct. 19, 2020, Evotec announced that its Seattle-based subsidiary, Just – Evotec Biologics had received a grant…

On Oct. 19, 2020, Mateon Therapeutics announced the receipt of approval from Republica Argentina Poder Ejecutivo Nacional, the…