NIH-funded study suggested COVID-19 increased risk of pregnancy complications
On Feb. 4, 2022, the NIH announced a study that showed pregnant women with COVID-19 appeared to be…

On Feb. 4, 2022, the NIH announced a study that showed pregnant women with COVID-19 appeared to be…

On Feb. 4, 2022, a new ᆪ1.6m collaborative project was launched to rapidly identify new treatments for COVID-19….

On Feb. 4, 2022, pet hamsters probably carried the Delta variant of SARS-CoV-2 into Hong Kong and sparked…

On Feb. 4, 2022, the Advisory Committee on Immunization Practices (ACIP) issued a standard recommendation for use of…

On Feb. 3, 2022, Novavax announced that the Medicines and Healthcare products Regulatory Agency had granted conditional marketing…

On Feb. 3, 2022, T2 Biosystems announced that its T2SARS-CoV-2 Panel detected Omicron COVID-19 subvariants BA.1, BA.2, and…

On Feb. 3, 2022, Novavax announced that New Zealand’s Medsafe had been granted provisional approval of NVX-CoV2373, Novavax’…
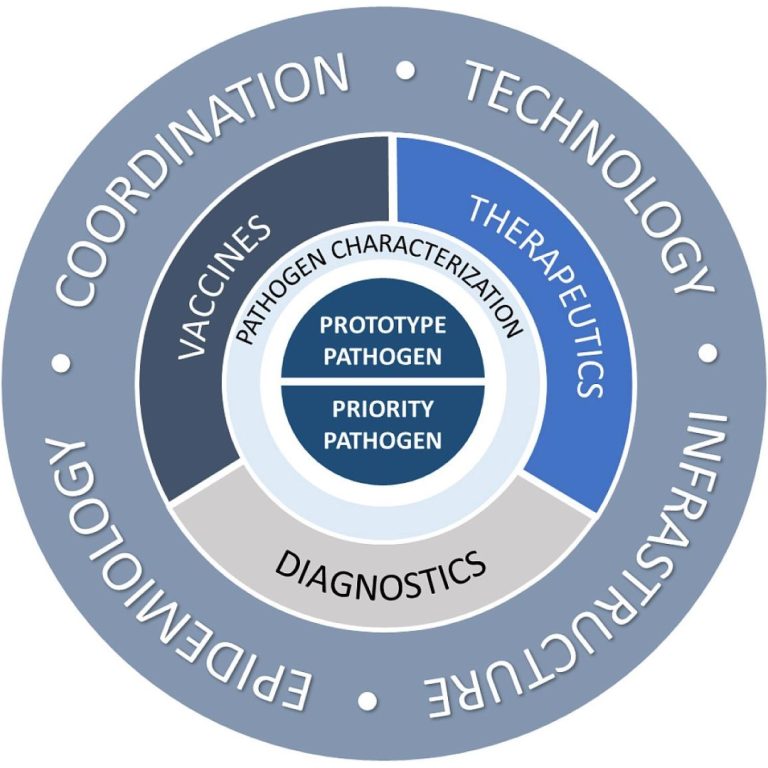
On Feb. 2, 2022, NIAID scientists announced the new Pandemic Preparedness Plan aimed to support critical basic and…

On Feb. 1, 2022, Pfizer and BioNTech announced that following a request from the U.S. Food and Drug…

On Jan. 31, 2022, Moderna announced the U.S. Food and Drug Administration (FDA) had approved the Biologics License…

On Jan. 31, 2022, Veru announced that the U.S. Food and Drug Administration (FDA) had granted Fast Track…

On Jan. 31, 2022, Novavax announced that it had submitted a request to the U.S Food and Drug…

On Jan. 31, 2022, OraSure Technologies announced that its InteliSwabᆴ COVID-19 rapid tests had been authorized by the…

On Jan. 28, 2022, Merck and Ridgeback Biotherapeutics announced data from six preclinical studies demonstrating that molnupiravir, an…

On Jan. 28, 2022, Novavax and Israel’s Ministry of Health announced an agreement for the purchase of NVX-CoV2373,…

On Jan. 28, 2022, the WHO announced that COVID-19 information had reached 1,292,209 people through Viamoメs 3-2-1 Platform…

On Jan. 27, 2022, Cocrystal Pharma announced that it had selected two investigational novel antiviral drug candidates for…
On Jan. 27, 2022, NIAID announced that a clinical trial found that the combination of remdesivir plus a…

On Jan. 26, 2022, the NIH announced that adults who had previously received a full regimen of any…

On Jan. 26, 2022, Moderna announced that the first participant had been dosed in the Phase 2 study…

On Jan. 25, 2022, researchers at the University of Missouri announced they had identified the highly prevalent, specific…
On Jan. 25, 2022, Cepheid announced that Health Canada had issued Cepheid a medical device license for Xpert…

On Jan. 25, 2022, Pfizer and BioNTech announced the initiation of a clinical study to evaluate the safety,…
On Jan. 24, 2022, Pfizer and BioNTech announced the publication of new results from two laboratory studies demonstrating…

On Jan. 24, 2022, Anixa Biosciences announced that the company and its partner, MolGenie, had synthesized a compound…
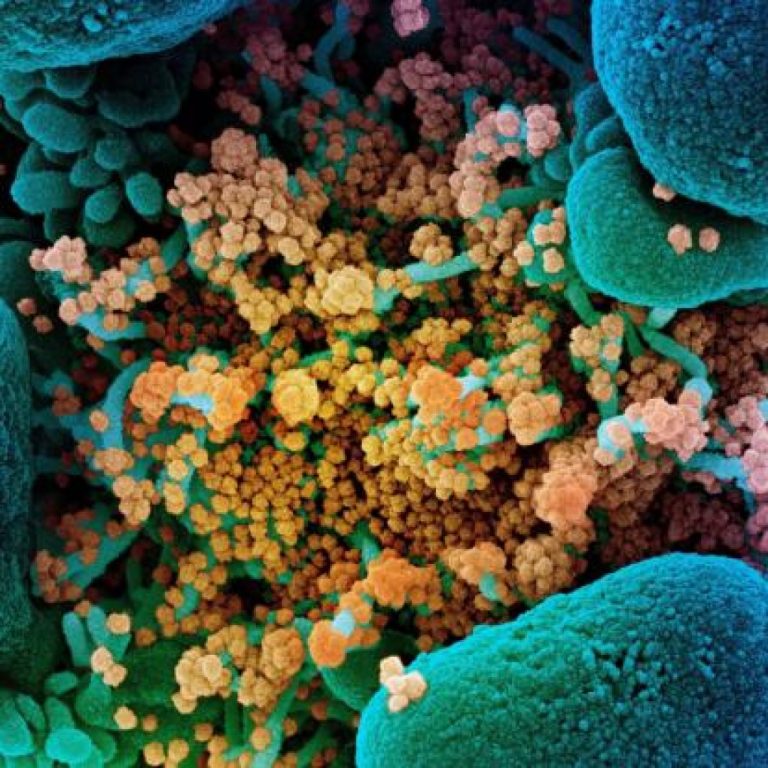
On Jan. 20, 2022, scientists from the National Institute of Allergy and Infectious Diseases (NIAID) announced that they…

On Jan. 20, 2022, Gilead Sciences announced that the U.S. Food and Drug Administration had granted expedited approval…

On Jan. 19, 2022, Novavaxa announced that Australia’s Therapeutic Goods Administration had granted approval for provisional registration of…

On Jan. 18, 2022, Sorrento Therapeutics announced receipt of clearance from the Brazilian regulatory agency (ANVISA) to proceed…

On Jan. 18, 2022, Merck and Ridgeback Biotherapeutics announced the signing of a long-term supply agreement with the…