UConn-made COVID-19 custom mask solution to be manufactured
On Jul. 2, 2020, UConn announced a licensing deal with Connecticut Biotech, a startup company headquartered in South…

On Jul. 2, 2020, UConn announced a licensing deal with Connecticut Biotech, a startup company headquartered in South…

On Jul. 2, 2020, Regeneron and Sanofi announced that the U.S. Phase 3 trial of Kevzara (sarilumab) 400…
On Jul. 2, 2020, the U.S. Food and Drug Administration (FDA) approved Rukobia (fostemsavir), a new type of…
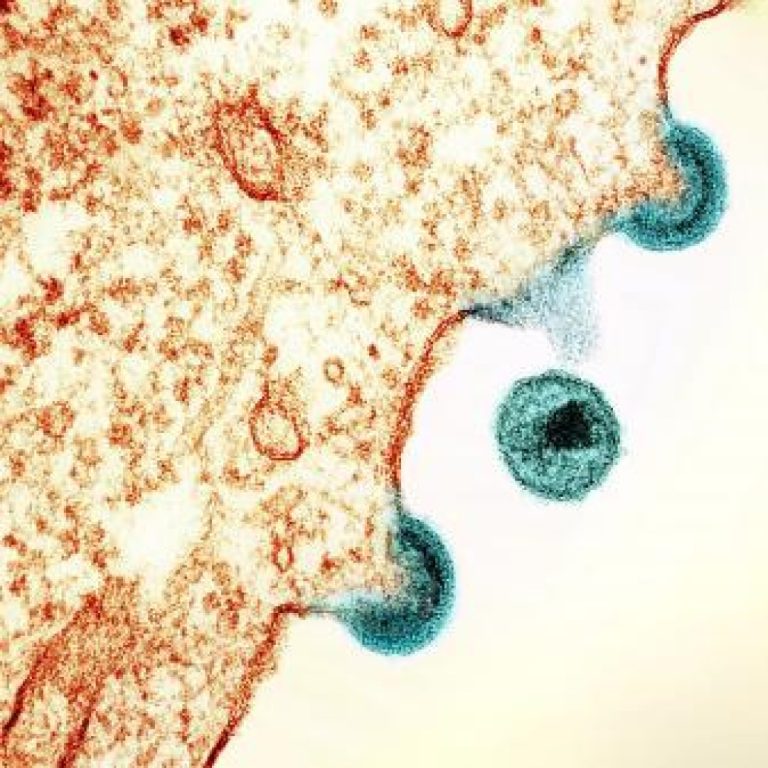
On Jul. 2, 2020, the U.S. Centers for Disease Control and Prevention (CDC) announced a publication in the…

On Jul. 2, 2020, scientists from Duke University, Los Alamos National Laboratory and La Jolla Institute for Immunology…

On Jul. 1, 2020, Intravacc announced the signing of a strategic partnership agreement with Dutch based CimCure, focusing…
On Jul. 1, 2020, National Institutes of Health (NIH) funded scientists announced using a combination of advanced microscopy…

On Jul. 1, 2020, InBios announced that it had received Emergency Use Authorization (EUA) from the FDA for…

On Jun. 30, 2020, AquaBounty Technologies announced it had successfully commenced the commercial-scale harvest of conventional Atlantic salmon…

On Jun. 30, 2020, Altimmune announced dosing of the first patient in the Company’s Phase 1b clinical trial…
On Jun. 29, 2020, National Institutes of Health (NIH) researchers announced they had discovered that Mediterranean populations may…

On Jun. 29, 2020, the Michael Smith Foundation for Health Research (MSFHR) COVID-19 Research Response Fund announced funding…

On Jun. 29, 2020, Luminex announced that the company had submitted an Emergency Use Authorization request to the…

On Jun. 26, 2020, the COVID-19 Therapeutics Accelerator donors and partners announced the formation of the International COVID-19…

On Jun. 26, 2020, the California Institute for Regenerative Medicine (CIRM) awarded $750,000 to Dr. Xiaokui Zhang at…

On Jun. 25, 2020, in support of Mayo Clinic’s digital health and practice transformation initiatives, the Mayo Clinic…
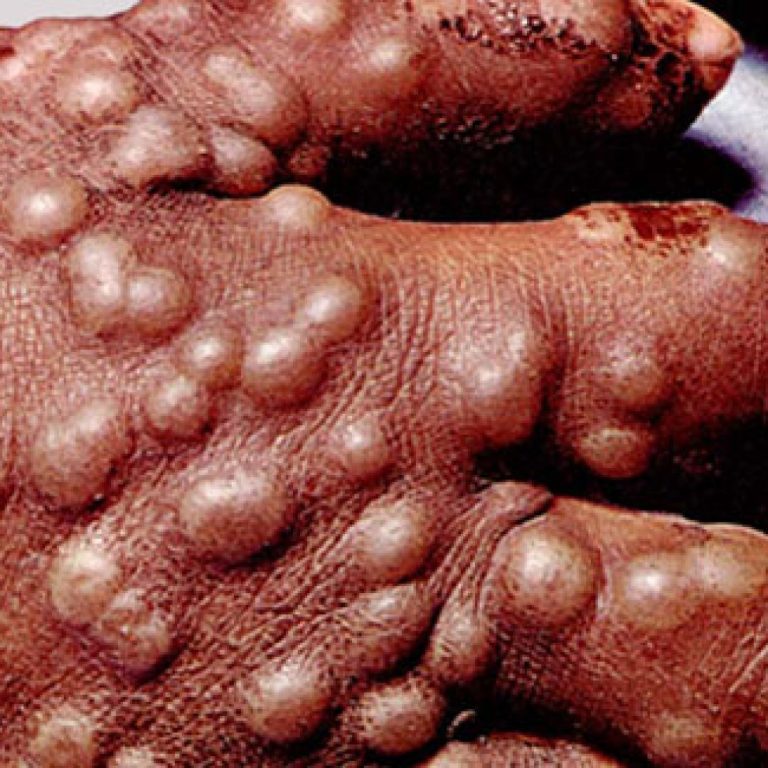
On Jun. 25, 2020, SIGA Technologies announced the deliveries of oral TPOXX (tecovirimat) to the U.S. Department of…
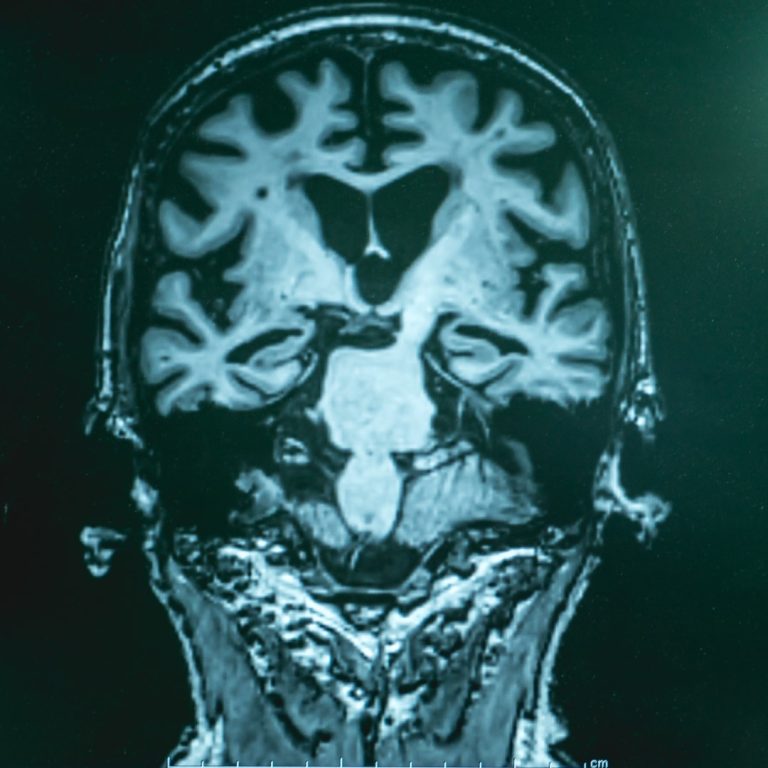
On Jun. 25, 2020, the Allen Institute announced a new a new $40.5 million collaborative research center in…

On Jun. 25, 2020, the University of Alberta (U of A) announced a study that will analyze thousands…

On Jun. 25, 2020, the U.S. Food and Drug Administration (FDA) approved Fintepla (fenfluramine), a Schedule IV controlled…

On Jun. 25, 2020, the Government of Canada announced the results of that funding competition: an investment of…

On Jun. 25, 2020, researchers at the University of British Columbia announced they had received a combined total…

On Jun. 25, 2020 Moderna announced a collaboration for large-scale, commercial fill-finish manufacturing of Moderna’s mRNA-based COVID-19 vaccine…
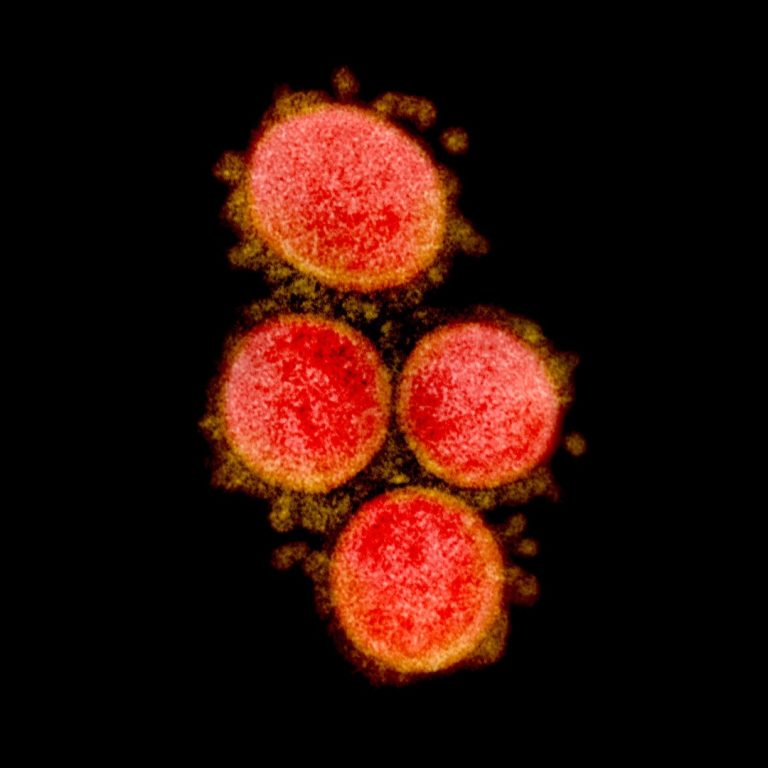
On Jun. 24, 2020, RedHill Biopharma announced that results from the treatment of the first severe COVID-19 patients…

On Jun. 24, 2020, Sinovac Biotech announced the China National Medical Products Administration (NMPA) issued a product license…

On Jun. 24, 2020, Oncotelic, a wholly owned subsidiary of Mateon Therapeutics, announced that IBM has granted access…

On Jun. 23, 2020, Mateon Therapeutics announced it had selected IQVIA to manage C001, a Phase 2 randomized,…

On Jun. 23, 2020, scientists at Scripps Research reported that some common strains of influenza have the potential…

On Jun. 23, 2020, Takeda Pharmaceutical announced that the FDA had approved the company’s submission for its biologics…

On Jun. 23, 2020, studies in the Netherlands and other places have shown that the presence of SARS-Coronavirus-2,…